| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website http://www.currentsurgery.org |
Original Article
Volume 9, Number 4, December 2019, pages 39-44
Comparison of 3% vs. 23.4% Hypertonic Saline in Traumatic Brain Injury
David Traficantea, Dina Galaktionovab, d, Urielle Marseillec, Steven Hochmana, Jamshed Zuberib, Robert Madlingerb
aDepartment of Emergency Medicine, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
bDepartment of Trauma Surgery, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
cNew York Medical College, Valhalla, NY 10595, USA
dCorresponding Author: Dina Galaktionova, Department of Trauma Surgery, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
Manuscript submitted October 10, 2019, accepted October 28, 2019
Short title: Comparison of 3% vs. 23.4% HTS in TBI
doi: https://doi.org/10.14740/jcs389
| Abstract | ▴Top |
Background: Hypertonic saline (HTS) is an effective treatment for patients with increased intracranial pressure (ICP) secondary to traumatic brain injury (TBI). The ideal concentration for use in these patients is not well defined. The aim of our study was to compare Glasgow coma scale (GCS) and mortality of patients after administration of 3% vs. 23.4% HTS in the initial resuscitation.
Methods: We performed a retrospective analysis of patients admitted to the surgical intensive care unit (ICU) under the trauma service with a diagnosis of TBI who received HTS during initial resuscitation. Patient medical records were reviewed to collect data including in-hospital mortality, ICU length of stay, hospital length of stay, GCS at the time of admission and discharge, serum sodium and serum osmolality values at 24, 48 and 72 h after arrival, acute kidney injury and severe hypernatremia.
Results: Patients ≥ 18 years of age admitted to trauma ICU with a diagnosis of TBI. Pregnant, incarcerated, or non-traumatic intracranial hemorrhage patients were excluded. Thirty-one patients were included in the study. The 3% arm included 21 patients, and 23.4% arm had 10 patients. All patients received 3% HTS continuous infusion following initial bolus. Median injury severity scores (ISS) were 22 vs. 25 in the 3% vs. 23.4% HTS groups, respectively (P = 0.37). There was no difference in in-hospital mortality between the two groups (52.4% vs. 50.0%, P = 0.45). There was a significant improvement in GCS at discharge, 8.3% vs. 44.4% in 3% HTS vs. 23.4% HTS arms, respectively (P = 0.029). Patients reaching goal serum sodium and serum osmolality at 24 h was significantly higher in the 23.4% group (33.3% vs. 70.0%; P = 0.028 and 35.7% vs. 77.8%; P = 0.026, respectively). Significant increase in incidence of severe hypernatremia in the 23.4% arm was noted (0.0% vs. 40.0%, P = 0.009).
Conclusion: This study demonstrates no significant difference in in-hospital mortality for patients who received 3% vs. 23.4% HTS. Significantly higher percentage of patients receiving 23.4% HTS reached goal serum sodium and osmolality levels at 24 h with a concomitant significantly increased rate of severe hypernatremia.
Keywords: Traumatic brain injury; Hypertonic saline therapy; Cerebral edema; Traumatic intracranial hemorrhage; Hospital mortality; Glasgow coma scale
| Introduction | ▴Top |
The use of hyperosmolar agents in the treatment of acute phase intracranial hypertension secondary to traumatic brain injury (TBI) has repeatedly been proven effective for controlling intracranial pressure (ICP) and diminishing the detrimental effects of secondary brain injury [1-3]. Currently, the American College of Surgeons recommends a “tiered approach” to management of intracranial hypertension. Tier 1 recommendations include head of bed elevation, sedation and analgesia and intermittent ventricular drainage for control of elevated ICP.
As a Tier 2 recommendation for elevated ICP (if pressure remains elevated > 20 - 25 mm Hg after Tier 1 approach), the ACS recommends use of hyperosmolar therapy, either mannitol or various concentrations of hypertonic saline (HTS), on an as-needed basis [4]. Historically, there has been much debate as to which hyperosmolar agent is more efficacious for lowering ICP and improving mortality for patients with elevated ICP secondary to TBI. However, more recent research appears to favor HTS [5-7]. For example, a recent meta-analysis by Li et al determined that HTS was more effective than mannitol for reducing ICP in cases of intracranial hypertension [8].
Although data continue to show the effectiveness of HTS, we are aware of only one prior study, which compared effectiveness of various concentrations of HTS (3% vs. 5%) in patients with acute TBI [9]. That particular study revealed sustained higher serum osmolarity and serum sodium concentrations within the first 72 h using 5% HTS compared to 3% HTS. There are currently no studies comparing the effectiveness of 3% vs. 23.4% HTS.
The key question of our study is what, if any, are the differences in clinical outcomes of patients receiving different HTS bolus concentrations (specifically 3% vs. 23.4%) in patients with computed tomography (CT) confirmed TBI and suspected increased ICP. Although the overall achieved serum osmolarity is significantly higher in the 23.4% HTS group compared to the 3% HTS group (8,008 mOsmol/L vs. 1,026 mOsmol/L), the mOsmol delivered per dose are relatively equal (240 mOsmol vs. 256 mOsmol). The difference in total osmolarity and osmolarity delivered per dose is due to the different total volumes of each solution given (30 mL bolus of 23.4% HTS vs. 250 mL bolus of 3% HTS). As such, we hypothesize that there will be no significant difference between the two groups in regards to our primary and secondary endpoints.
| Materials and Methods | ▴Top |
This study was an IRB-approved retrospective analysis that was conducted at an academic urban level II trauma center. Patients included in the study were admitted under the trauma service to a 12-bed surgical intensive care unit and were managed by a multidisciplinary team led by trauma surgeons.
Patients eligible for inclusion were identified using the Trauma Registry of the American College of Surgeons (TRACS) during a 3-year period, from January 1, 2013 to December 31, 2015. Patients were identified by searching for the admitting and/or discharge diagnosis (and accompanying ICD code) including the terms “traumatic brain injury”, “subarachnoid hemorrhage”, “subdural hematoma”, “epidural hematoma” and “intracerebral hemorrhage”. Patients must have had a confirmed diagnosis of TBI via CT scan and were included if they received a bolus of 3% or 24.3% HTS within 24 h of arriving in the emergency department (ED). All patients must have also received 3% HTS as a continuous infusion after the initial bolus. Exclusion criteria included age < 18 years old, pregnant patients, incarcerated patients and patients with intracranial bleeds not secondary to trauma (i.e. those patients with intracranial pathology secondary to brain tumor or spontaneous hemorrhagic stroke).
Demographic data including patient age, sex, ethnicity, admission Glasgow coma scale (GCS) values and injury severity score (ISS) were collected using TRACS. Additional data including the TBI subtype for each patient, incidence of midline shift and/or herniation as well as vitals and lab values were obtained from each patient’s medical record. The intracerebral hemorrhage (ICH) score for each individual patient was calculated from data collected from each medical record.
The primary outcomes of the study were in-hospital mortality as well as percentage of patients meeting goal serum sodium and serum osmolality levels within 24 h. Time zero was the documented time of arrival in the ED. Goal serum sodium values for each individual patient were determined and documented by the neurosurgical team involved in each patient’s care. The range for goal serum sodium levels was typically between 150 and 160 mEq/L. Goal serum osmolality value was defined as a serum osmolality ≥ 310 mOsm/kg. Secondary outcomes included ICU length of stay, hospital length of stay, GCS at the time of discharge compared to initial GCS, serum sodium and serum osmolality values at time 24, 48 and 72 h. In addition, safety outcomes included incidence of acute kidney injury and hypernatremia (Na ≥ 160 mEq/L). GCS values are designated as mild (13 - 15), moderate (9 - 12) and severe (3 - 8) based on the American College of Surgeons (ACS) and Eastern Association for the Surgery of Trauma (EAST) guidelines. Acute kidney injury was defined as an increase in serum creatinine of greater than or equal to 1.5 times baseline serum creatinine value, according to the RIFLE criteria [10].
| Results | ▴Top |
A total of 363 trauma patients were reviewed, of which 31 were included in the study (21 patients received 3% HTS bolus and 10 patients received 23.4% HTS bolus). Patient demographics were relatively similar between the two groups (Table 1).
 Click to view | Table 1. Demographics |
There was no significant difference in injury severity score (22 (10 - 26) vs. 25 (18.3 - 25); P = 0.37) or intracranial hemorrhage score (median of 1.0 (0.0 - 3.0) for the 3% HTS group vs. median of 1.5 (1.0 - 3.0) for the 23.4% group; P = 0.15) between the two groups. However, the 3% HTS group did have a significantly higher initial ED GCS compared to the 23.4% HTS group (11.0 (6.0 - 14.0) vs. 4.0 (3.0 - 8.8); P = 0.02) and were less likely to undergo immediate neurosurgical intervention (28.6% vs. 40.0%; P = 0.27), although this was not statistically significant (Table 1).
There was no statistical significant difference in in-hospital mortality between the two groups (52.4% vs. 50.0%, P = 0.45) (Fig. 1). There was also no statistical difference in both ICU length of stay in days (7.0 (5.3 - 11.3) vs. 10.0 (5.0 - 15.0); P = 0.23) and hospital length of stay in days (12.5 (10.3 - 15.0) vs. 17.0 (11.0 - 19.0); P = 0.087). The GCS of surviving patients at the time of discharge was similar in both groups (14.5 (14.0 - 15.0) vs. 15.0 (14.0 - 15.0); P = 0.34) (Table 2).
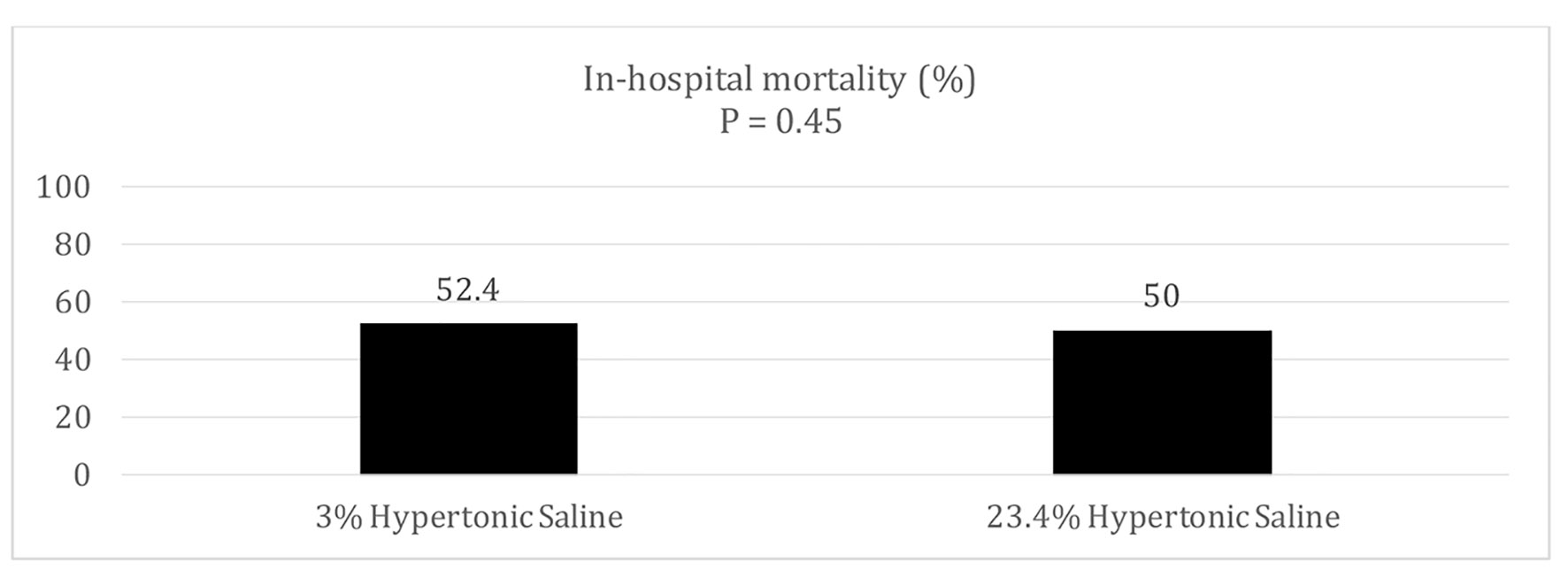 Click for large image | Figure 1. In-hospital mortality. |
 Click to view | Table 2. Secondary Outcomes |
The initial sodium value in the ED (mEq/L) was comparable for both groups (median of 135.0 (132 - 138) for the 3% HTS group vs. median of 134.5 (133 - 138.3) for the 23.4% HTS group; P = 0.36). At 24 h, however, the 23.4% HTS group had a significant higher serum sodium concentration (median of 144.0 (140.0 - 146.0) for the 3% HTS group vs. median of 150.0 (147.5 - 153.3) for the 23.4% HTS group; P = 0.015), higher percentage of patients reaching goal serum sodium values (33.3% vs. 70.0%; P = 0.028) and goal serum osmolality values (35.7% vs. 77.8%; P = 0.026) compared to the 3% HTS group (Fig. 2). This was at the expense, however, of a clinically significant increase in incidence of severe hypernatremia in the 23.4% cohort (0.0% vs. 40.0%, P = 0.009).
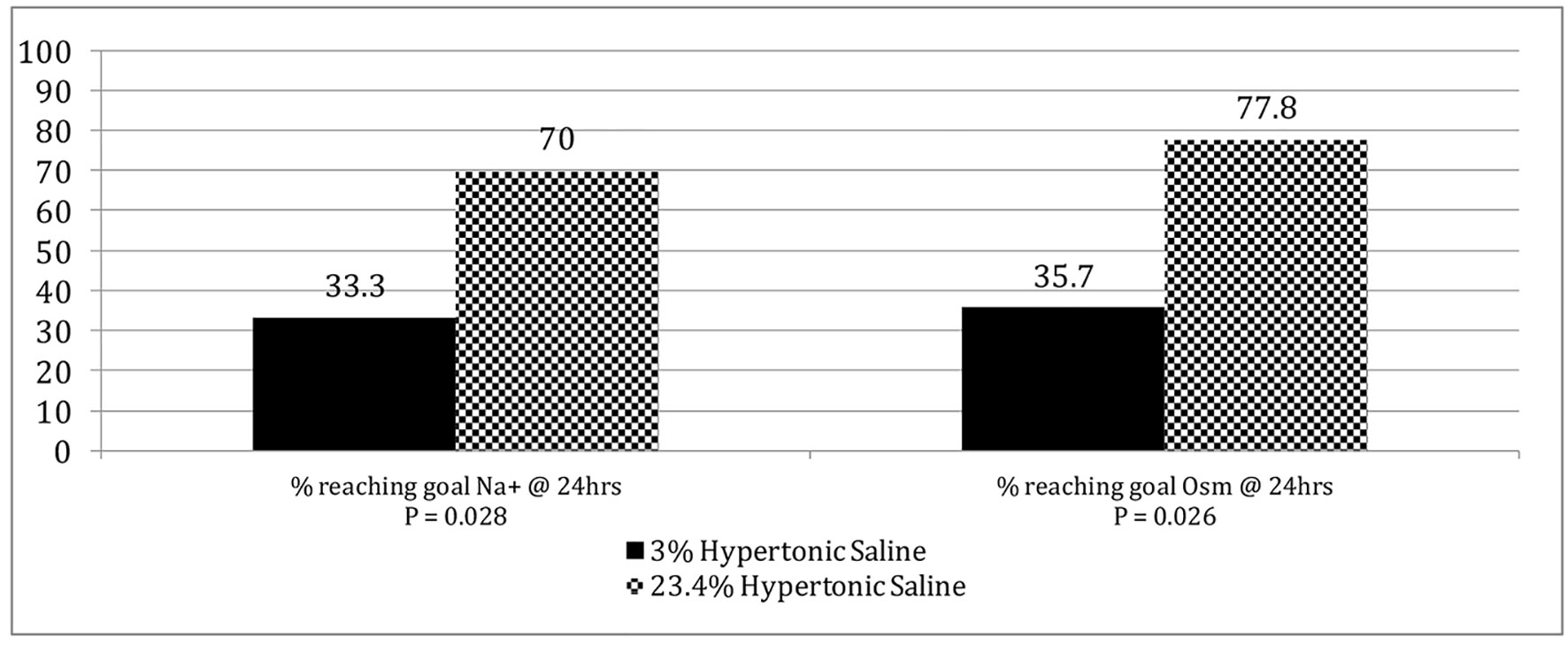 Click for large image | Figure 2. Percentage of patients reaching goal sodium and serum osmolality values at 24 h. |
Of patients who presented to the ED with moderate (9 - 12) or severe (3 - 8) GCS scores, we compared the percentage of each cohort that improved to a mild (13 - 15) GCS score at the time of discharge. There was a clinically significant increase with the 23.4% HTS group with our finding that 8.3% vs. 44.4% (P = 0.029) of these patients improved from a moderate or severe GCS (3 - 12) at presentation to a mild GCS (13 - 15) at the time of discharge.
| Discussion | ▴Top |
In this retrospective non-randomized study, we compared the clinical outcomes and differences between patients with suspected elevated ICP secondary to TBI who received either 3% or 23.4% HTS during their initial resuscitation. The authors are aware of no other studies comparing the effectiveness of these two commonly used concentrations of HTS. The primary outcome of the study demonstrates no significant difference in in-hospital mortality between the two groups. It is important to point out that there was a trend towards patients with worse initial GCS values to receive 23.4% HTS. Although we did not find any significant change in mortality, based on improvement in GCS values there appears to be a clinically significant improvement in morbidity with the use of 23.4% HTS. Because of the small non-randomized sample size, as well as the inherent limitations of being a retrospective study, the data should be taken with a grain of salt. However, the authors believe the data warrant additional prospective studies in the future to further evaluate our conclusions.
The study is also notable for two other important findings. A higher proportion of patients in the 23.4% HTS group reached goal serum sodium and serum osmolality levels at 24 h with the caveat that these patients were also more likely to develop severe hypernatremia. As more recent research appears to favor HTS, this has become our first-line hyperosmolar agent for patients with suspected increased ICP. Physiologically, it is the increased concentration of sodium intravascularly that leads to the osmotic shift and decreased ICP. For all patients receiving hyperosmolar therapy, we closely monitor their serum sodium and serum osmolality values. Although Wells et al described poor correlation between serum sodium value and ICP, we continue to monitor this value in patients receiving hyperosmolar therapy to be cautious of severe or extreme elevations in serum sodium [9].
In fact, a safety outcome in our study revealed a clinically significant increase in the percentage of patients receiving 23.4% HTS who developed severe hypernatremia, which was defined as sodium greater than or equal to 160 mEq/L. These severe elevations in serum sodium levels may lead to fatal arrhythmias, acute kidney injury, altered mental status, seizures, coma and even death [11, 12]. Similar to mannitol, higher concentrations of HTS in animal studies, specifically 23.4% HTS, have also shown to have diuretic effects [13].
Another safety outcome of our study revealed an increase in percentage of patients that developed acute kidney injury for those patients who received 23.4% HTS. Acute kidney injury was defined as an increase in serum creatinine more than 1.5 times baseline, per the RIFLE criteria. Although this increase was not clinically significant, a study may be warranted with increased number of patients to further explore this side effect [10, 14]. Goal serum osmolality for our study was chosen to be ≥ 310 mOsm/kg, based on previous studies, which demonstrated improved outcomes for patients who reached a value of 310 - 320 mOsm/kg [15]. Because the calculated mOsmol delivered per dose is fairly equal between 3% and 23.4% HTS, as previously described, we were surprised to see the significant finding that patients in the 23.4% HTS group reached goal serum osmolarity values more rapidly than those patients in the 3% HTS group at 24 h.
There are several limitations of our study. First and foremost, our study is a retrospective analysis, which has inherent limitations by design. In regards to concentration, amount and delivery (bolus or continuous infusion), there is currently no standardized protocol for the delivery of hyperosmolar therapy in our hospital. Because of these differences and the need to study patients receiving HTS in the same manner, we were limited in the study’s sample size with a total number of 31 patients. In addition, there are some important differences in the patient demographics between the two groups including admission GCS and percentage of patients undergoing neurosurgical intervention. These differences may have contributed to the choice of which HTS concentration to use and outcomes that we observed in our study. Finally, although all of the included patients had suspected elevated ICP secondary to their injury, another limitation was the lack of reported ICP pressures data points for each patient. Therefore, patients may have received hyperosmolar therapy without having truly clinically significant elevations in their ICP.
Conclusion
This study compared the outcomes and differences between patients with suspected elevated ICP secondary to TBI who received 3% or 23.4% HTS during their initial resuscitation. Patients who received 23.4% HTS were more likely to reach goal serum sodium and serum osmolality levels at 24 h with the caveat that there was also a higher rate of severe hypernatremia. In addition, there was no significant difference in in-hospital mortality for patients who received 3% or 23.4% HTS. This study adds important data regarding the comparison of using 3% or 23.4% HTS in patients with suspected elevated ICP from TBI. While this study suffers from the limitations of a retrospective non-randomized design, it points to the need for a randomized controlled trial to compare the efficacy of 3% vs. 23.4% HTS in this patient population.
Acknowledgments
We thank Saint Joseph’s University Medical Center Trauma Division.
Financial Disclosure
This study was conducted under the guidance and support of Department of Trauma and Department of Emergency Medicine. No other funding was provided to conduct this study.
Conflict of Interest
All authors have no conflict of interest to declare. This research did not receive any grant from funding agencies in the public, commercial or not-for-profit sectors.
Informed Consent
Not applicable.
Author Contributions
Research idea: Jamshed Zuberi, MD, MPH, FACS; Steven Hochman, MD; Robert Madlinger DO, FACOS, FACS. Data collection: David Traficante, DO; Dina Galaktionova, DO, FACOS; Urielle Marseille, MD. Data analysis: David Traficante, DO; Jamshed Zuberi, MD, MPH, FACS. Manuscript author: David Traficante, DO. Manuscript review: Dina Galaktionova, DO, FACOS; Steven Hochman, MD; Jamshed Zuberi, MD, MPH, FACS; Robert Madlinger, DO, FACOS, FACS. Corresponding author: Dina Galaktionova, DO, FACOS.
| References | ▴Top |
- Ropper AH. Hyperosmolar therapy for raised intracranial pressure. N Engl J Med. 2012;367(8):746-752.
doi pubmed - Vassar MJ, Fischer RP, O'Brien PE, Bachulis BL, Chambers JA, Hoyt DB, Holcroft JW. A multicenter trial for resuscitation of injured patients with 7.5% sodium chloride. The effect of added dextran 70. The multicenter group for the study of hypertonic saline in trauma patients. Arch Surg. 1993;128(9):1003-1011; discussion 1011-1003.
doi pubmed - Oddo M, Levine JM, Frangos S, Carrera E, Maloney-Wilensky E, Pascual JL, Kofke WA, et al. Effect of mannitol and hypertonic saline on cerebral oxygenation in patients with severe traumatic brain injury and refractory intracranial hypertension. J Neurol Neurosurg Psychiatry. 2009;80(8):916-920.
doi pubmed - American College of Surgeons, Committee on Trauma, trauma quality improvement program best practices; Traumatic brain injury guidelines. Intracranial pressure monitoring; management of intracranial hypertension. 2015:6-11.
- Brain Trauma F, American Association of Neurological S, Congress of Neurological S, Joint Section on N, Critical Care AC, Bratton SL, Chestnut RM, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37-44.
- Huang XC, Yang LL. [Comparison clinical efficacy of 3% hypertonic saline solution with 20% mannitol in treatment of intracranial hypertension in patients with aneurysmal subarachnoid hemorrhage]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44(4):389-395.
- Kerwin AJ, Schinco MA, Tepas JJ, 3rd, Renfro WH, Vitarbo EA, Muehlberger M. The use of 23.4% hypertonic saline for the management of elevated intracranial pressure in patients with severe traumatic brain injury: a pilot study. J Trauma. 2009;67(2):277-282.
doi pubmed - Li M, Chen T, Chen SD, Cai J, Hu YH. Comparison of equimolar doses of mannitol and hypertonic saline for the treatment of elevated intracranial pressure after traumatic brain injury: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(17):e736.
doi - Wells DL, Swanson JM, Wood GC, Magnotti LJ, Boucher BA, Croce MA, Harrison CG, et al. The relationship between serum sodium and intracranial pressure when using hypertonic saline to target mild hypernatremia in patients with head trauma. Crit Care. 2012;16(5):R193.
doi pubmed - Luo X, Jiang L, Du B, Wen Y, Wang M, Xi X, Beijing Acute Kidney Injury Trial w. A comparison of different diagnostic criteria of acute kidney injury in critically ill patients. Crit Care. 2014;18(4):R144.
doi pubmed - Arambewela MH, Somasundaram NP, Garusinghe C. Extreme hypernatremia as a probable cause of fatal arrhythmia: a case report. J Med Case Rep. 2016;10(1):272.
doi pubmed - Kumar AB, Shi Y, Shotwell MS, Richards J, Ehrenfeld JM. Hypernatremia is a significant risk factor for acute kidney injury after subarachnoid hemorrhage: a retrospective analysis. Neurocrit Care. 2015;22(2):184-191.
doi pubmed - Toung TJ, Nyquist P, Mirski MA. Effect of hypertonic saline concentration on cerebral and visceral organ water in an uninjured rodent model. Crit Care Med. 2008;36(1):256-261.
doi pubmed - Joseph B, Aziz H, Snell M, Pandit V, Hays D, Kulvatunyou N, Tang A, et al. The physiological effects of hyperosmolar resuscitation: 5% vs 3% hypertonic saline. Am J Surg. 2014;208(5):697-702.
doi pubmed - Wagner I, Hauer EM, Staykov D, Volbers B, Dorfler A, Schwab S, Bardutzky J. Effects of continuous hypertonic saline infusion on perihemorrhagic edema evolution. Stroke. 2011;42(6):1540-1545.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
