| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 10, Number 3, September 2020, pages 32-36
Rare Presentation of a Krukenberg Tumor
Reshad Salama, d, Henrik Ghantarchyana, Stephanie Yeeb, Jamshed Zuberib, Essam Ahmadc
aSt. Joseph University Medical Center, 703 Main St., Paterson, NJ 07503, USA
bDepartment of Surgery, St. Joseph University Medical Center, 703 Main St., Paterson, NJ 07503, USA
cDepartment of Pathology, St. Joseph University Medical Center, 703 Main St., Paterson, NJ 07503, USA
dCorresponding Author: Reshad Salam, St. Joseph University Medical Center, 703 Main St., Paterson, NJ 07503, USA
Manuscript submitted April 14, 2020, accepted May 15, 2020, published online August 22, 2020
Short title: Rare Presentation of a Krukenberg Tumor
doi: https://doi.org/10.14740/jcs407
| Abstract | ▴Top |
A Krukenberg tumor is a rare form of metastatic ovarian cancer. Distinctly identified by its histological appearance, a signet-ring cell, it is an uncommon and aggressive neoplasm commonly seen in pre-menopausal women. We present a rare case of a Krukenberg tumor seen in a 36-year-old Mexican female with no known past medical history. Treatment modalities for our patient included metastasectomy followed by multiple rounds of chemotherapy.
Keywords: Krukenberg tumor; Ovarian cancer; Metastatic gastric adenocarcinoma; Signet-ring cell gastric carcinoma; Surgery; Immunostains; Metastasis; Prognostic factors
| Introduction | ▴Top |
Krukenberg tumor is a rare form of metastatic ovarian cancer histologically characterized by signet-ring adenocarcinoma with a primarily gastrointestinal source. The condition is named after the German physician Friedrich Ernst Krukenberg (1871 - 1946) who first described five cases in 1896 [1]. The pathology accounts for 1-2% of all ovarian tumors, but in countries with a high incidence of gastric cancer, e.g. Japan, Korea, China, the prevalence is much higher [2].
Most patients present with bilateral ovarian involvement (80%), and the prognosis is very poor with a median survival of only 14 months [2]. A Krukenberg tumor can present at any age; however, the average age of diagnosis is 40 - 46 years [2]. This report presents an unusual case of a Krukenberg tumor in a 36-year-old healthy young female and highlights the diagnostic workup undertaken.
| Case Report | ▴Top |
A 36-year-old Mexican female with no significant past medical history presented to the emergency department complaining of intermittent episodes of epigastric abdominal pain with occasional bouts of diffuse abdominal pain. Reportedly, the pain was worse at night with laying down, along with consumption of food. As these pain episodes were of first occurrence, the patient was prescribed famotidine by her primary care physician, which prevailed to no relief. A further workup included an ultrasound of the abdomen, which revealed ascites of the anterior abdomen. Findings from an abdominal and pelvic computed tomography with contrast revealed a complex 4.5 cm right ovarian mass (Fig. 1) and moderate ascites with nodular heterogenous omentum suspicious for carcinomatosis/peritonitis. The liver, pancreas, spleen, adrenal glands and kidneys were normal. There was no retroperitoneal adenopathy noted and the bowel was free of obstruction. Consecutively, the blood serum levels of CA125 level were 267 U/mL on the day of presentation, and subsequent measurement 5 days later revealed a persistent elevation of CA125 of 279 U/mL with a CA19-9 of 8.
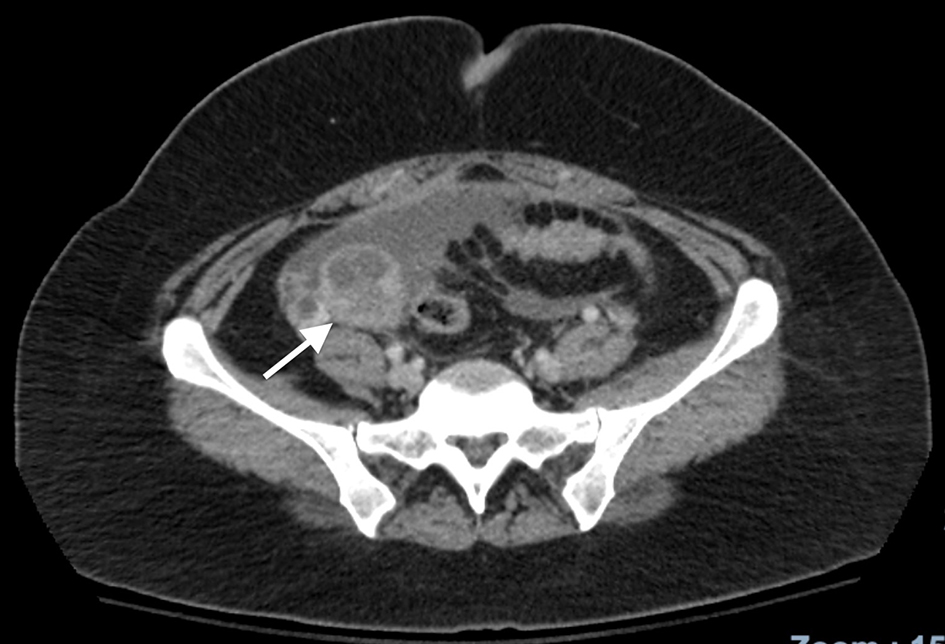 Click for large image | Figure 1. CT scan of the abdomen characteristic for a right ovarian tumor. CT: computed tomography. |
The decision was made to perform a diagnostic laparoscopy with total abdominal hysterectomy, bilateral salpingo-oophorectomy and omentectomy. Results from the procedure revealed 4 L of straw colored ascitic fluid, small bowel matted in a large cluster, and edematous ascending, transverse, and descending colon. The transverse colon was noted to be adherent to the greater curvature of the stomach and there was obliteration of the lesser sac. The omentum was shrunken and hard, the ovaries appeared enlarged, approximating at 5 - 6 cm in size bilaterally with papillary growths and firm in texture.
The frozen section biopsy of the ovary was obtained and pathology analysis revealed signet-ring cell carcinoma (Krukenberg tumor) involving the bilateral ovaries, fallopian tubes and omentum (Fig. 2). To aid in distinguishing the source of malignancy, immunohistochemical analysis was commissioned. Immunophenotype expression of markers found on epithelial membranes and intestinal protein was employed to distinguish a primary ovarian carcinoma from a possible metastatic gastric source. The ovarian sample was found to be positive for cytokeratin 7 (CK7), CK20 and CDX2 immunoprofile, consistent with a gastric primary origin. This strengthened the diagnosis of metastatic adenocarcinoma of the ovary over primary ovarian carcinoma. Esophagogastroduodenoscopy (EGD) was performed to assess a possible gastric source. Findings from the EGD revealed prominent and edematous gastric folds, multiple (four) superficial clean-based ulcers with raised and scalloped edges in the distal gastric body. Gastric biopsies were taken from the antrum, body and the friable mucosa surrounding the gastric ulcers. Biopsies from the gastric ulcers treated with Alcian blue/periodic acid-Schiff (PAS) staining exhibited intercytoplasmic mucin in signet-ring cells, supporting the diagnosis of signet-ring cell carcinoma (Fig. 3). Further immunostaining was negative for H. pylori. Human epidermal growth factor receptor 2 (Her-2) neu determination was negative for the gastric ulcer specimen (Fig. 4). The diagnosis of Krukenberg tumor was made and management for the malignancy was commenced by the oncology department. Three months later, a decrease in hemoglobin was noted. This was evidenced by an EGD which revealed duodenal erosions, a gastric ulcer with a clean base, hematin in the stomach and an acquired duodenal stenosis. Oral intake was poorly tolerated, and the patient’s primary mode of nutrition was via total parenteral nutrition (TPN). As the patient’s condition escalated quickly, the decision was made to transition to hospice care. The patient deceased after 1 day of hospice care.
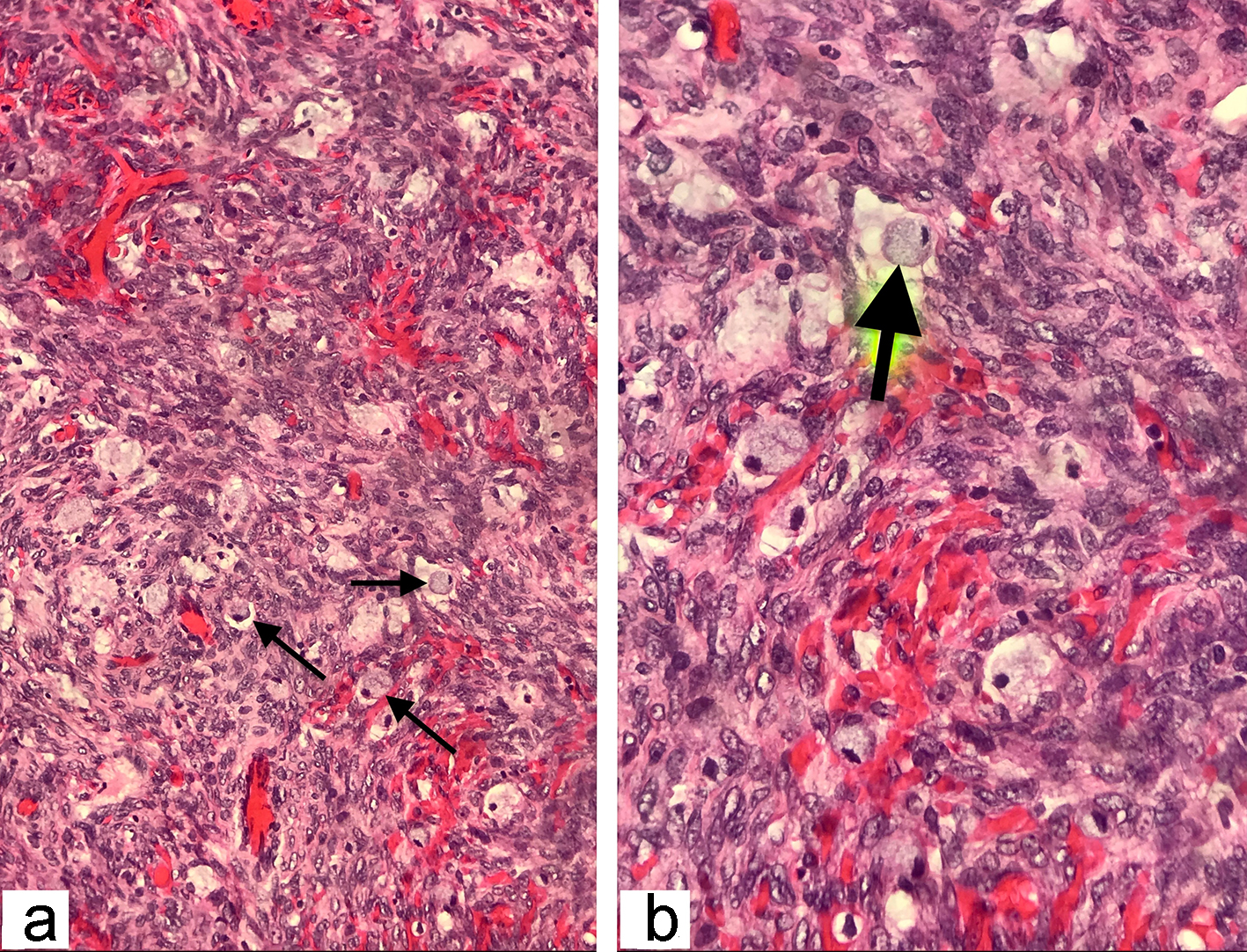 Click for large image | Figure 2. The histopathological examination of the frozen section ovarian-biopsy showing poorly differentiated, diffusely infiltrating signet ring cell carcinoma. Arrows pointing towards the signet ring cells, containing large mucin filled intracytoplasmic vacuoles, with eccentric hyperchromatic nuclei pushed towards the periphery (hematoxylin and eosin staining; magnification: (a) × 200 and (b) × 400). |
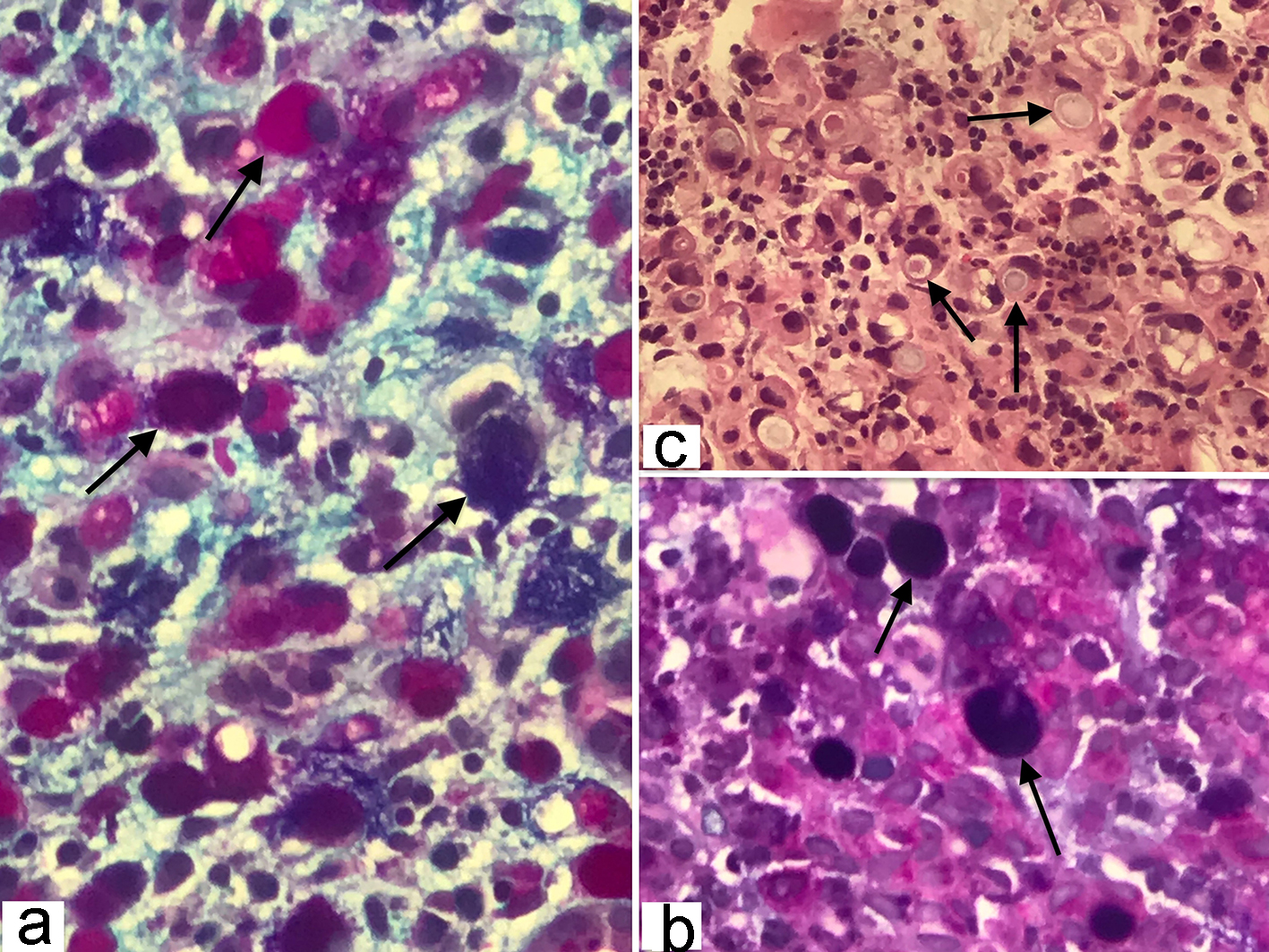 Click for large image | Figure 3. (a, b) Gastric signet-ring cell carcinoma characterized by the presence of abundant intracytoplasmic mucin, combination of Alcian blue (pH 2.5) and periodic acid-Schiff (PAS) stain color the neutral and acid mucins demonstrating a dark blue/purple or magenta coloration (original magnification, × 400). (c) Histopathology of gastric adenocarcinoma showing mucin filled vacuoles that push the nucleus to one side, as shown by the arrows, characteristic of signet ring cell pattern (hematoxylin and eosin, × 200). |
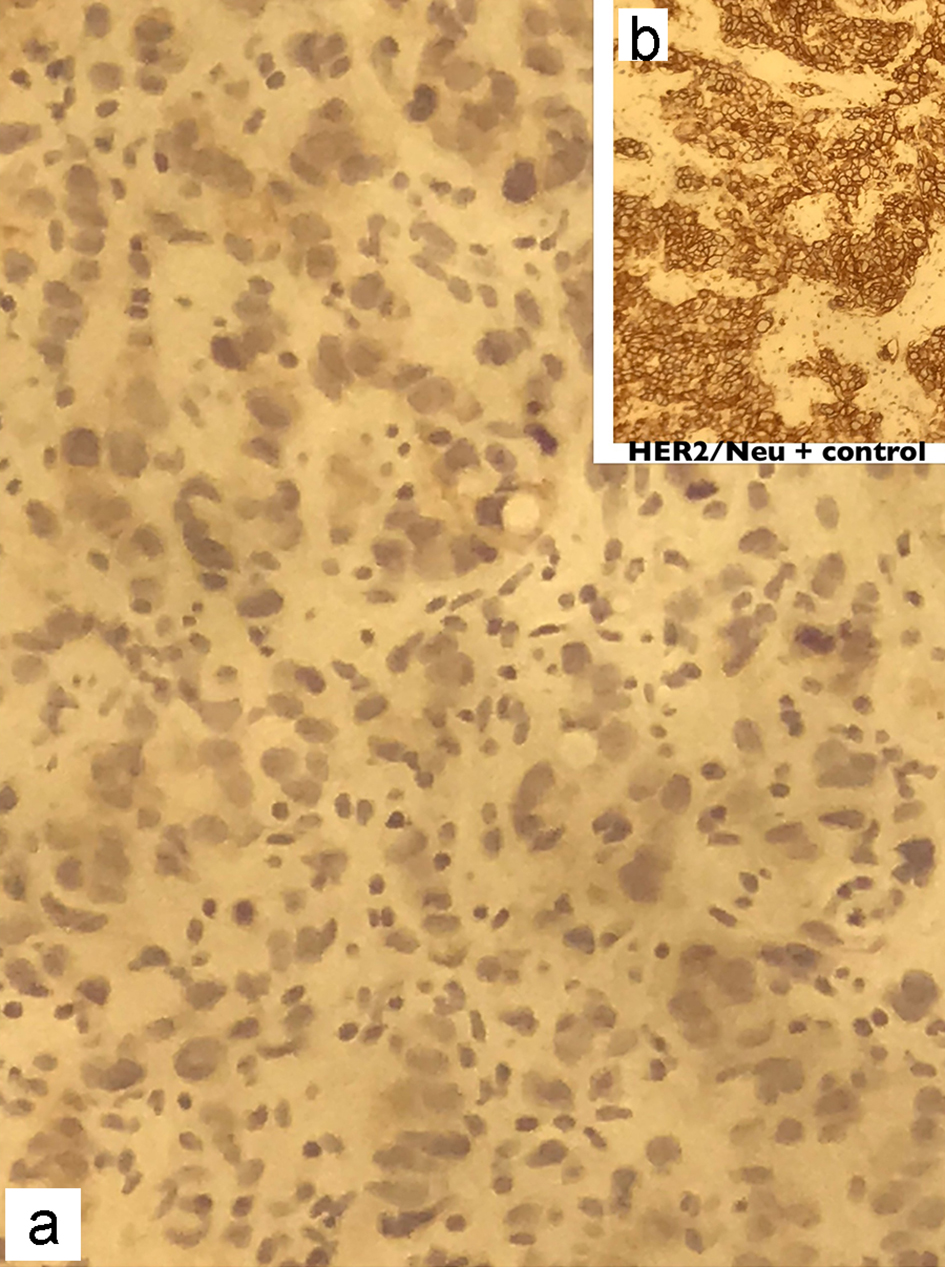 Click for large image | Figure 4. Immunohistochemical staining of the gastric biopsy with Her-2/Neu monoclonal antibodies showing absence of membranous staining in the patient (a), compared to the strong continuous membranous staining in positive control (b). Her-2: human epidermal growth factor receptor 2. |
| Discussion | ▴Top |
In the majority of cases, Krukenberg tumors seem to originate from a gastric or colorectal source but there have been reports made in the past suggesting metastasis form other sites such as the small intestine, breast, pancreas, biliary tract and gallbladder [3, 4]. Krukenberg tumor predominantly affects pre-menopausal women, with the average age of diagnosis at 40 - 46 years. A possible explanation for this is that the functioning ovary has a rich blood supply which is more prone to hematogenous metastasis [2]. Also, some researchers believe that the rupture of the ovary due to ovulation releases growth factors as part of the wound healing process which promotes cell migration into the wound area, known as chemotaxis. This could potentially attract metastatic tumor cells and lead to ovarian malignancy [2].
The mechanism of metastasis has not been established yet; however, there are three possible pathways of dissemination discussed in literature: lymphatic, hematogenous and peritoneal [5].
Our patient only endorsed symptoms of abdominal pain and nausea, and these non-specific complaints could be easily mistaken for more commonly occurring gastrointestinal disorders such as non-ulcer dyspepsia or gastritis. Krukenberg tumors can present with wide-ranged and non-specific symptoms; however, the most common presenting signs are abdominal pain and distention, explained by the enlargement of the ovaries [4].
The current World Health Organization diagnostic criteria for Krukenberg Tumor are based on the pathological description by Serov and Scully. It states that to make a diagnosis the following features should be present: 1) Presence of stromal involvement; 2) Ovarian stromal sarcomatoid proliferation; 3) Presence of mucin-producing signet-ring cells [2].
The patient in our case was initially found to have characteristic signet-ring cells present on biopsy in the ovaries, fallopian tubes and omentum. The first step was to determine whether the malignancy was a primary ovarian malignancy or due to metastasis. Immunohistochemical testing is commonly employed to guide the physician in determining the source of ovarian carcinomas. Primary ovarian malignancy tends to be highly immunoreactive for the CK7 (90-100%) immunophenotype and negative for CK20. Metastatic gastric carcinoma on the other hand is generally less positive for CK7 (55%) but shows positivity for CK20. Tumor metastasis from a colorectal source is generally negative for CK7 but positive for CK20 [4].
Our patient’s immunophenotype showed immunoreactivity for both CK7 and CK20, and this prompted us to search for a gastric malignancy as the source. Serum CA125 levels are useful to track in patients affected by this disorder, it is used post-operatively to assess the success of resection of the tumor and it can also be used to screen patients with pre-existing gastrointestinal, breast or other malignancies for possible ovarian metastasis [4].
Prognosis of the disease is poor and depends on the source of the metastasis with median survival of 11, 21.5, 31 and 19.5 months for gastric, colorectal, breast and other origins, respectively [3]. A recent meta-analytic study by Lionetti et al looked at the clinicopathologic factors affecting the prognosis in Krukenberg tumor. Twenty-three studies with 1,743 patients were included and the result showed that a decreased overall survival (OS) was significantly associated with ascites (hazard ratio (HR) 2.055; P = 0.034), synchronous presentation (HR 1.679; P = 0.034), increased serum carcinoembryonic antigen (CEA) levels (HR 1.380; P = 0.01000) and peritoneal involvement (HR 1.944; P = 0.003) [6].
There is a lack of consensus regarding the treatment strategy for this disease: no curative treatment is available but given the poor survival rate it is of utmost importance for the physician to choose one that will optimize survival time even if it is by a few months.
There are a few different treatment options available such as cytoreductive surgery (CRS), adjuvant chemotherapy (CT) and hyperthermic intraperitoneal chemotherapy (HIPEC). Cytoreduction is also referred to as debulking and describes the process of surgically removing tumors. HIPEC is a type of chemotherapy which employs thermal injury to induce heat-shock protein release from tumor cells and facilitates cytotoxicity of chemotherapeutic medication [7].
In our patient, the debulking procedure was completed to decrease pain and obstructive symptoms, and improve the activities of daily living, thus globally promoting a better quality of life. Although the patient perished shortly after the operation (3 months), we believe this was due to her extensive metastatic disease at the time of presentation. We cannot confidently claim that metastasectomy lengthened the patient’s life but based on the limited available literature, it seems to be the best option available. A systematic review of 20 retrospective studies on the association of OS by Lionetti et al concluded that CRS without residual and HIPEC seem to be effective in prolonging OS with CT showing conflicting evidence [8]. Two additional studies report that metastasectomy shows a significantly longer median OS compared to those who did not undergo the same procedure. Cheong et al reported a median 17-month OS in the resection cohort compared to 3 months in the non-resection group who were managed with either systemic chemotherapy or supportive care [9]. Studies by Lu et al and Jiang et al also reported a prolonged OS in patients who underwent metastasectomy [10, 11].
In the case of a diagnosis of Krukenberg tumor, it is of importance that physicians consider inspecting for possible extra-ovarian sources. As this cancer is known to exhibit aggressive expansion and metastasis, early metastasectomy may prolong median survival time, thus promoting an improved quality of life with increasing functioning of daily living. An important treatment modality is HIPEC, a mode of chemotherapy in which thermal injury is employed to induce heat-shock protein release from tumor cells. This promotes cytotoxicity of chemotherapeutic medication.
Conclusion
We present a rare case of a metastatic ovarian cancer known as a Krukenberg tumor. It is highlighted by its distinct histological appearance, known as a signet-ring cell. A very poor outcome with a median survival of only 14 months is important to consider in the diagnosis and management of this tumor [2]. The mainstay of treatment in our case included chemotherapy and surgery.
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
RS and HG participated in the patient care. RS and HG wrote the case report. EA, JZ and SY revised and edited the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chandradas D, Thomas B, Jayachandran A. Diagnosing and treating Krukenberg tumor: a gynecologist's dilemma. Int J Reprod Contracept Obstet Gynecol. 2017;4(6):2069-2071.
doi - Shah B, Tang WH, Karn S. Transcoelomic spread and ovarian seeding during ovulation: A possible pathogenesis of Krukenberg tumor. J Cancer Res Ther. 2017;13(1):152-153.
doi pubmed - Aziz M, Kasi A. Cancer, Krukenberg Tumor. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482284/.
- Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006;130(11):1725-1730.
- Agnes A, Biondi A, Ricci R, Gallotta V, D'Ugo D, Persiani R. Krukenberg tumors: Seed, route and soil. Surg Oncol. 2017;26(4):438-445.
doi pubmed - Lionetti R, De Luca M, Travaglino A, Raffone A, Saccone G, Di Cicco A, Insabato L, et al. Prognostic factors in Krukenberg tumor. Arch Gynecol Obstet. 2019;300(5):1155-1165.
doi pubmed - Chen LY, Fu CY, Lu HE, Chan DC. Treatment of Krukenberg tumor with hyperthermic intraperitoneal chemotherapy: A report of three cases. J Med Sci. 2016;36:197-201.
doi - Lionetti R, De Luca M, Travaglino A, Raffone A, Insabato L, Saccone G, Mascolo M, et al. Treatments and overall survival in patients with Krukenberg tumor. Arch Gynecol Obstet. 2019;300(1):15-23.
doi pubmed - Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol. 2004;94(2):477-482.
doi pubmed - Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, Cheng AL, et al. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012;32(8):3397-3401.
- Jiang R, Tang J, Cheng X, Zang RY. Surgical treatment for patients with different origins of Krukenberg tumors: outcomes and prognostic factors. Eur J Surg Oncol. 2009;35(1):92-97.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
