| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 10, Number 3, August 2020, pages 37-40
A Case Report of Likely Nosocomial-Acquired COVID-19 in a Trauma Patient
Naina Raoa, c, Clive Persaudb, Stephanie Yeeb, Manrique Guerrerob, Jamshed Zuberib, Robert Madlingerb
aNew York Medical College, Valhalla, NY 10595, USA
bDepartment of Surgery, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
cCorresponding Author: Naina Rao, New York Medical College, Valhalla, NY 10595, USA
Manuscript submitted April 22, 2020, accepted May 11, 2020, published online August 12, 2020
Short title: Nosocomial-Acquired COVID-19
doi: https://doi.org/10.14740/jcs409
| Abstract | ▴Top |
As the pandemic of coronavirus disease 2019 (COVID-19) continues to spread worldwide, there has been an increase in unique clinical presentations leading to delayed diagnoses and nosocomial transmissions. One of the patient populations most at risk includes patients in the critical care units. Early diagnosis and isolation are paramount to avoid nosocomial transmission amongst these closely hospitalized patients. While quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) is the current method for testing, we highlight the importance of utilizing chest computed tomography (CT) and laboratory findings for early diagnosis. We report a 48-year-old trauma patient who suddenly became hypoxemic, nine days postoperative from uncomplicated right lower extremity fracture repair. CT angiogram chest revealed bilateral extensive consolidations, hazy opacities, and pleural effusions. The patient continued to desaturate on noninvasive respiratory support and eventually required intubation. He was empirically treated with azithromycin and hydroxychloroquine due to high clinical suspicion of COVID-19, despite negative qRT-PCR results. The patient progressed clinically and was successfully extubated after 5 days. This unique presentation of acute hypoxemic respiratory failure warrants a discussion on the importance of clinical manifestations, CT findings, and laboratory findings in diagnosis of COVID-19, to prevent further nosocomial spread within a closed critical care unit.
Keywords: Acute hypoxemic respiratory failure; COVID-19; Nosocomial
| Introduction | ▴Top |
Since December 2019, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes coronavirus disease 2019 (COVID-19) has rapidly spread worldwide and overwhelmed the capacity of many hospitals. It has been shown that infection with COVID-19 can be fatal with development of acute respiratory distress syndrome and multiple organ failure [1]. As our understanding of COVID-19 continues to grow, we have learned about factors that make nosocomial spread difficult to prevent. Some of these challenges include variable clinical presentations, contagiousness even when asymptomatic, and prolonged incubation periods [2]. Furthermore, the current method of testing via quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) makes rapid detection challenging due to delayed results and possible false negatives [3]. Currently the complete understanding of nosocomial spread, especially in the surgical intensive care unit, is not well defined. Given its contagiousness and the severity of illness this respiratory virus can cause, it is important to review the elements of diagnosis. Several studies have shown that chest imaging and laboratory markers are important tools for early detection and classification of disease severity [1-3]. We discuss a case of a trauma patient who initially presented with minimal chest CT and chest X-ray (CXR) findings related to his motor vehicle accident and then suddenly desaturated 9 days after hospitalization. The patient was diagnosed with nosocomial-acquired COVID-19 based on new extensive consolidations and hazy opacities seen on repeat imaging in combination with elevated inflammatory markers. Through rapid early detection the patient was quickly placed in an airborne isolation room, managed carefully, and made full recovery despite the necessity of invasive respiratory support.
| Case Report | ▴Top |
A 48-year-old African American male with a history of insulin-dependent diabetes mellitus was brought to the emergency room after a motor vehicle collision that caused multiple right lower extremity fractures and required surgical intervention. On days 2 and 7 in hospital, respectively, the patient underwent open reduction and internal fixation (ORIF) of right subtrochanteric femur fracture and ORIF of right bicondylar acetabular fracture. He was recovering in the surgical intensive care unit, with six COVID-19 positive patients in adjacent rooms.
Seven days after his operation (day 9 in hospital) he was found to be acutely hypoxemic with a peripheral oxygen saturation (SpO2) of 41% on room air. He denied shortness of breath and only reported new onset chest pain with deep breathing. On exam, the patient was alert, afebrile, tachycardic, tachypneic, with moderate accessory muscle usage and had diminished breath sounds bilaterally on auscultation. He was given 6 L/min of supplemental oxygen via nasal cannula but continued to desaturate to the high 70% SpO2. Subsequently, a venturi mask was placed which improved his SpO2 to 90%. An arterial blood gas on oxygen showed partial pressure of oxygen (PaO2) 67 mm Hg and partial pressure of carbon dioxide (PaCO2) 45 mm Hg. CXR demonstrated bilateral pulmonary infiltrates which were not seen on admission CXR (Fig. 1). A CT angiogram chest demonstrated new extensive consolidations and hazy opacities involving bilateral lungs with new moderate-sized bilateral pleural effusions (Fig. 2). Laboratory data showed white blood cell count of 11.8 × 103 cells/mm3 (88.2% neutrophils, 7.3% lymphocytes, neutrophil to lymphocyte ratio (NLR) of 12.08) and significantly raised inflammatory markers: erythrocyte sedimentation rate 122 mm/h (reference range (RR): 0 - 10 mm/h), C-reactive protein 311.3 mg/L (RR: < 9.9 mg/L), procalcitonin 4.43 ng/mL (RR: < 2.00 ng/mL), D-dimer 9.11 µg/mL (RR: < 0.50 µg/mL), and ferritin 1,132 ng/mL (RR: 16.4 - 294 ng/mL).
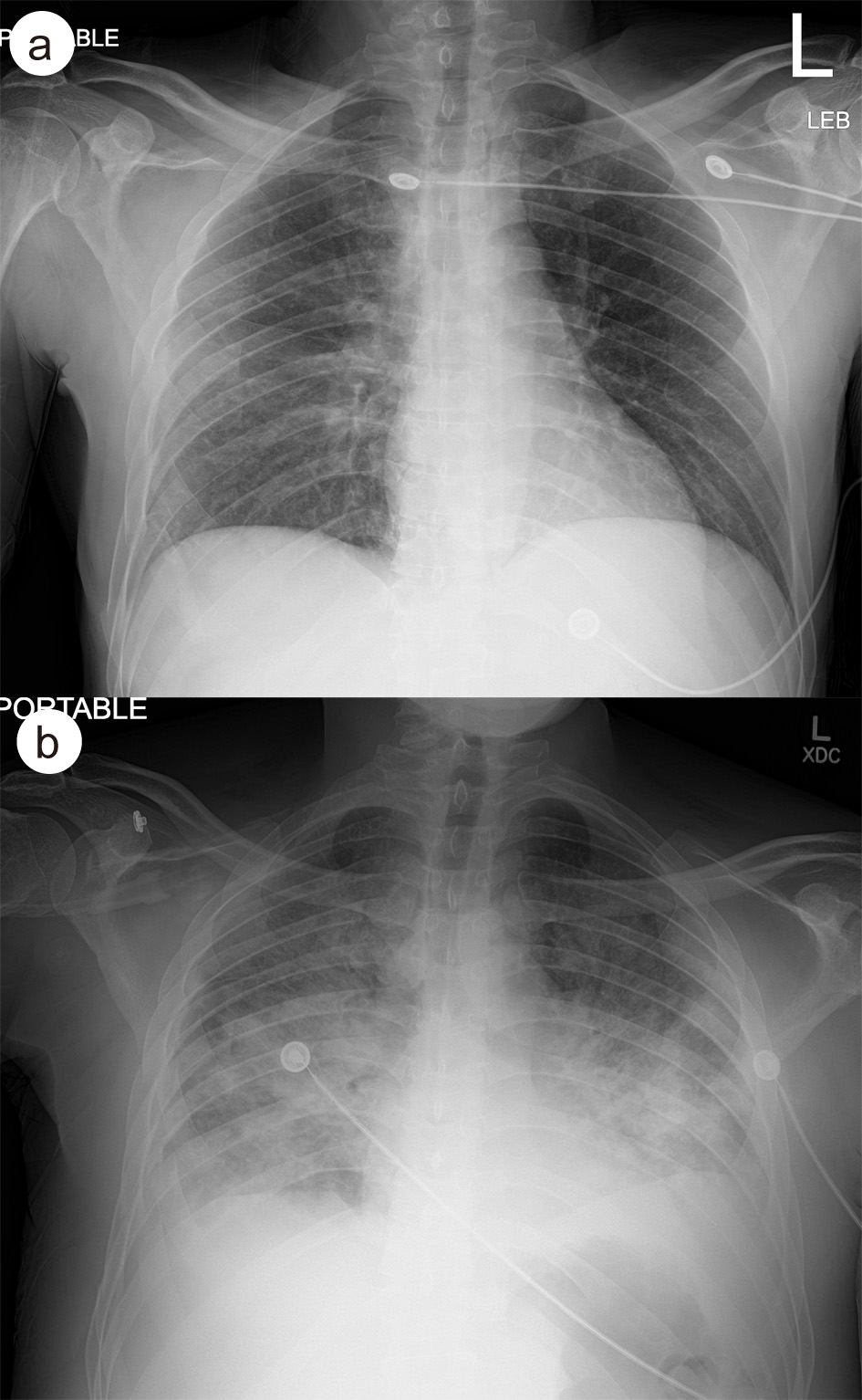 Click for large image | Figure 1. (a) Admission CXR showing hazy infiltrative change in the right midlung field. (b) CXR on day 9 in hospital, demonstrating diffuse hazy and more confluent airspace disease with small bilateral pleural effusions. CXR: chest X-ray. |
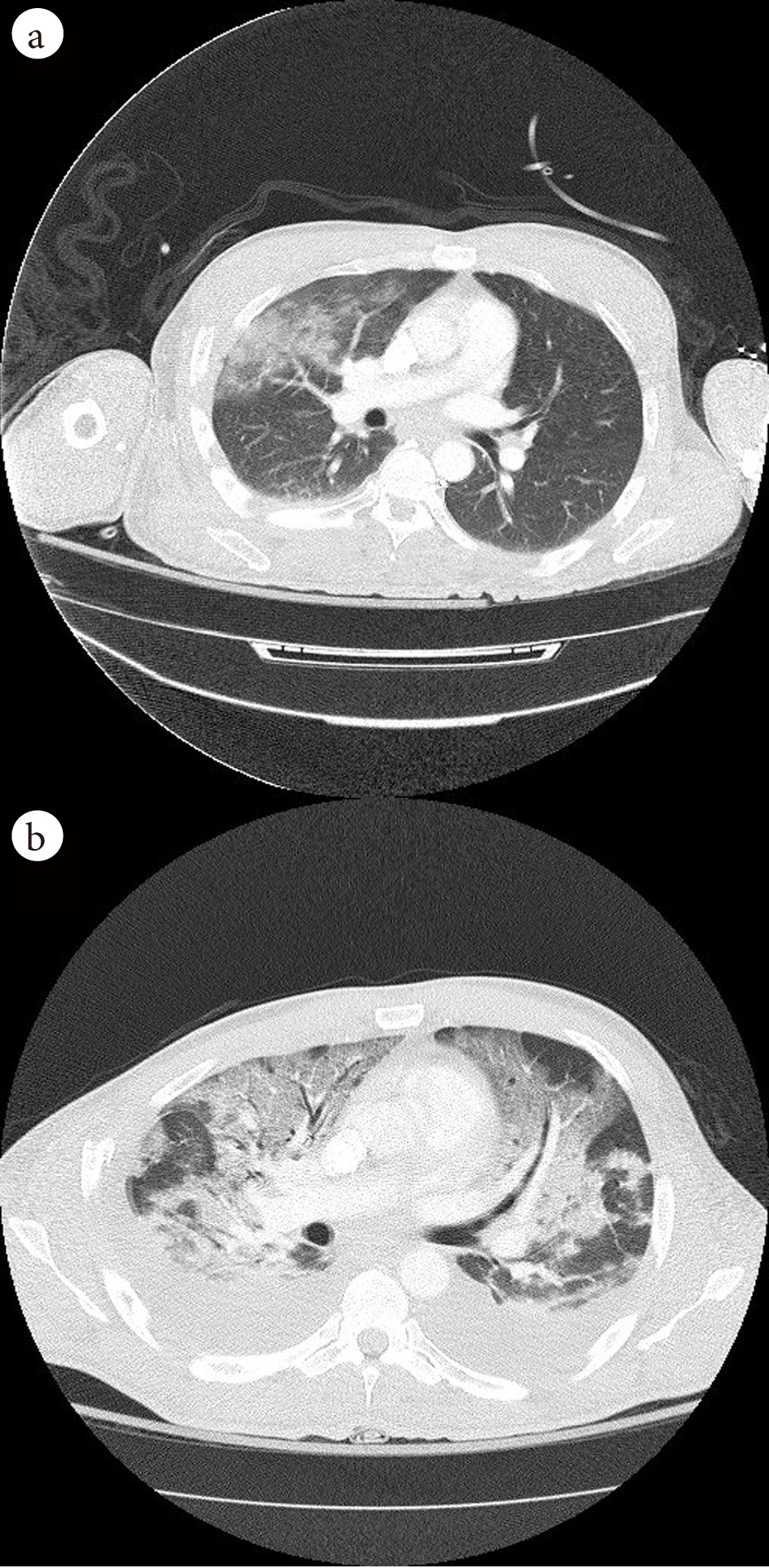 Click for large image | Figure 2. (a) Admission CT chest showing patchy airspace opacities in the right upper, middle and lower lobes. (b) CT chest on day 9 in hospital, demonstration of extensive consolidations and hazy opacities involving bilateral lungs with new moderate-sized bilateral pleural effusions. |
Given the patient’s clinical presentation during the COVID-19 outbreak, CT findings, and elevated inflammatory markers, a nasopharyngeal swab was obtained for SARS-CoV-2 qRT-PCR testing. He was empirically started on oral azithromycin 250 mg daily, oral hydroxychloroquine 200 mg twice daily, and intravenous (IV) ceftriaxone 1000 mg daily for suspected sepsis with acute hypoxemic respiratory failure due to possible COVID-19 infection.
On day 10 in hospital, the patient continued to deteriorate and was endotracheally intubated. Soon after, his SARS-CoV-2 result was found to be negative. However, given the patient's clinical presentation consistent with current known presentations of COVID-19 and the likelihood of a possible false negative result, the patient continued the current treatment regimen with azithromycin and hydroxychloroquine for a total 5-day course. Over the following next 3 days (days 12 - 14 in hospital), the patient’s respiratory status improved. Peep was gradually weaned from 15 mm Hg. By day 14 of hospitalization, the patient was successfully extubated and transferred to an isolation room where he stayed until discharge.
| Discussion | ▴Top |
Patients with COVID-19 can rapidly develop hypoxemia and dyspnea which can lead to hypoxemic respiratory failure, often necessitating intubation and intensive care management [1, 4]. The inconsistency in presenting symptoms and presence of minimally symptomatic to asymptomatic patients with COVID-19 has made early diagnosis complicated. Delayed diagnosis is particularly dangerous in critical care units caring for trauma patients where the possibility of nosocomial transmission can exist. Early diagnosis has not only shown to improve mortality rate [5] but can also decrease nosocomial spread through rapid initiation of airborne isolation. This is important in a surgical intensive unit where patients with traumatic injuries already have an increased risk of mortality. This case emphasizes the utility of chest imaging and laboratory markers to make a rapid clinical diagnosis in hospitalized patients with atypical presentations and avoid exposure to other patients in a surgical critical care unit.
Chest imaging is a key tool in evaluating patients with suspected COVID-19 infection. In our case report, we highlight the difference in chest CT and CXR findings at admission compared to time of desaturation. The rapid development of extensive consolidations and hazy opacities involving bilateral lungs in a relatively asymptomatic trauma patient warrants the discussion of nosocomial COVID-19 infection. In a study of 121 patients with confirmed COVID-19 infection, CT findings showed presence of either ground-glass opacities or consolidation in 78% of patients, and bilateral involvement in 60% of patients [6]. While these findings are consistent with what was seen in chest imaging of our patient immediately after sudden desaturation, they were not present during the patient’s initial admission. We therefore determined that our patient likely acquired COVID-19 nosocomially with rapid disease progression, given noncontributory chest imaging on admission, and close proximity to six COVID-19 positive patients during post-op recovery in the surgical intensive care unit. While the sensitivity for detection of COVID-19 in CXR is reported to be lower than that of CT, the widespread availability makes it a useful tool for screening [7]. The most common CXR findings of bilateral lung consolidation and ground glass opacities correlate with those found on chest CT [7, 8]. In our case study, these findings were only seen on CXR taken at the time of desaturation, again demonstrating the likelihood of nosocomial acquired infection. An additional finding noted on both CXR and chest CT of the patient we presented, were bilateral pleural effusions. While this finding has been rare on chest imaging of COVID-19 positive patients, some studies have identified pleural effusion late in the disease course [9]. Given that the patient continued to desaturate with supplemental oxygen and required intubation, pleural effusions could be a unique indicator of disease severity.
Laboratory findings can also be a useful tool for evaluating patients with suspected COVID-19. They are readily available and can aid in classifying disease severity of COVID-19. Studies have demonstrated increased inflammatory response in patients with severe infection, including elevated white blood cell counts, neutrophil counts, lower lymphocyte counts, and increased C-reactive protein levels [10, 11]. These findings were similarly seen in our patient at time of desaturation and improved in congruity with resolution of chest imaging findings. A study by Tan et al, found lymphopenia to be a predictor of disease severity, with lymphocytes < 20% to indicate severe disease [12]. Another useful laboratory marker is NLR, which increases with severity of infection and can be used as a prognostic factor [11]. In our patient the significantly elevated NLR at time of desaturation could explain the disease severity and aid in providing an early diagnosis despite a negative qRT-PCR test. The use of inflammatory markers to support diagnosis and classify disease severity should be considered for early detection and rapid initiation of airborne precautions.
The use of qRT-PCR in evaluation of patients with suspected COVID-19 can have its limitations. Currently, the primary modality of testing via nasopharyngeal swab for qRT-PCR has restrictions due to availability of test kits and limited sensitivity of only 71% [3]. A series of case reports have demonstrated the importance of imaging in early diagnosis of patients with negative qRT-PCR results. One case report revealed a patient with a close contact of confirmed COVID-19, presenting with a cough, and was admitted into an airborne isolation room after CXR showed an alveolar opacity in the left middle lung. The patient was found to have inconclusive results on qRT-PCR testing on admission day 1 and positive results on admission day 4 [13]. Another case report revealed a patient with four sequential negative qRT-PCR tests who was managed as a presumptive COVID-19 diagnosis based on chest CT findings of patchy ground-glass opacity and was later found to have a positive qRT-PCR on his fifth test [14]. Our case also demonstrates another example of diagnosing COVID-19 based on other elements of clinical evaluation. This further suggests that qRT-PCR results should not be the determining factor when evaluating the necessity of airborne precautions as this could challenge containment. However, it must be noted that repeat qRT-PCR testing was not done in our patient and would have been useful to confirm suspicion of a false negative result and further support diagnosis. Due to limited availability of qRT-PCR kits and delayed resulting time, repeat testing continues to be a challenge.
In conclusion, to better control nosocomial transmission of COVID-19, early detection in hospitalized patients is of most vital importance. When qRT-PCR results are pending or negative, further understanding of chest imaging and laboratory markers can aid diagnosis and classification of disease severity. With rapid diagnosis, early initiation of airborne precautions can help prevent a nosocomial outbreak, particularly in the critical care unit where time is of the essence.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
None to declare.
Author Contributions
NR wrote the manuscript with input from all authors. NR, CP, SY, MG, JZ, and RM edited the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513.
doi - Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514-523.
doi - Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. 2020;296(2):E115-E117.
doi pubmed - Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020.
doi pubmed - Sun Q, Qiu H, Huang M, Yang Y. Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province. Ann Intensive Care. 2020;10(1):33.
doi pubmed - Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295(3):200463.
doi pubmed - Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology. 2020;296(2):E72-E78.
doi pubmed - Jacobi A, Chung M, Bernheim A, Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): A pictorial review. Clin Imaging. 2020;64:35-42.
doi pubmed - Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87-93.
doi pubmed - Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020.
doi pubmed - Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504.
doi pubmed - Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Wang Q, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33.
doi pubmed - Sivakorn C, Luvira V, Muangnoicharoen S, Piroonamornpun P, Ouppapong T, Mungaomklang A, Iamsirithaworn S. Case report: walking pneumonia in novel coronavirus disease (COVID-19): mild symptoms with marked abnormalities on chest imaging. Am J Trop Med Hyg. 2020;102(5):940-942.
doi pubmed - Feng H, Liu Y, Lv M, Zhong J. A case report of COVID-19 with false negative RT-PCR test: necessity of chest CT. Jpn J Radiol. 2020;38(5):409-410.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
