| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 10, Number 3, September 2020, pages 41-44
Gastrobronchial Fistula: A Rare Complication Post-Laparoscopic Sleeve Gastrectomy
Ashen Fernandoa, c, Jean Luc Francoisa, Nicole Majachania, Reshad Salama, Stephanie Yeeb, Jamshed Zuberib
aSt. George’s University, True Blue, Grenada, West Indies
bDepartment of Surgery, St. Joseph’s University Medical Center, 703 Main St., Paterson, NJ 07503, USA
cCorresponding Author: Ashen Fernando, St. George’s University, True Blue, Grenada, West Indies
Manuscript submitted May 6, 2020, accepted May 28, 2020, published online August 22, 2020
Short title: GBF Post-LSG
doi: https://doi.org/10.14740/jcs413
| Abstract | ▴Top |
A gastrobronchial fistula (GBF) is an abnormal connection between the stomach and the lungs, and is an extremely rare but serious complication of laparoscopic sleeve gastrectomy (LSG). GBFs are usually the result of a persistent staple line leak that leads to the formation of a subphrenic abscess. The abscess may either spread through lymphatics or directly erode into the diaphragm and result in a GBF. We present the case of a 49-year-old female who developed a GBF after being managed for recurrent staple line leaks post-LSG. This case highlights the importance of timely detection and management of leaks to prevent this potentially fatal sequela.
Keywords: Gastrobronchial fistula; Laparoscopic sleeve gastrectomy; Bariatric surgery
| Introduction | ▴Top |
Laparoscopic sleeve gastrectomy (LSG) has become a well-established surgical approach in the management of morbid obesity. It promotes weight loss by reducing the capacity of the stomach and by altering hormone levels responsible for hunger and satiety. When compared to more invasive bariatric procedures, LSG is relatively simple, has a lower post-operative morbidity, and preserves the natural continuity of the gastrointestinal system [1]. Complications include strictures, staple line bleeding and staple line leakage [1-4]. Of these, staple line leakage carries the highest morbidity and occurs in up to 5.5% of primary cases [1, 3-7] and 20% of revisional cases [2]. Most leaks occur in the proximal stomach near the gastroesophageal junction [2]. A persistent gastric leak can increase the risk of forming a gastrobronchial fistula (GBF) which is a rare but potentially fatal complication [3, 8]. Minimally invasive endoscopic procedures with percutaneous drainage have been increasingly adapted to manage post-operative staple line leaks with success rates of 80-95% [1, 2, 4, 7]. However, leaks may become persistent despite intervention and progress to develop a GBF as in the case presented.
| Case Report | ▴Top |
A 49-year-old Hispanic female with a past medical history of asthma and morbid obesity underwent a LSG. One month post-operatively after LSG, the patient presented with left upper quadrant abdominal pain, vomiting and intolerance to oral intake. Laboratory studies were notable for a white blood cell (WBC) count of 22,000 cells/mm3, and a computerized tomography (CT) scan revealed three distinct fluid collections in the left upper quadrant compatible with abscesses and worrisome for a contained leak. Upper gastrointestinal endoscopy revealed a proximal staple line deformity. Lavage yielded copious purulent fluid through the defect, and it was closed using argon plasma ablation and an over-the-endoscope clip. The patient had an upper gastrointestinal series (UGIS) prior to discharge which did not demonstrate contrast extravasation. However, 1 month after the clip was placed, the patient returned to the emergency department with recurrent left upper abdominal pain that was worse with oral intake. Routine labs showed a WBC count of 15,500 cells/mm3, and a CT scan revealed extraluminal air with reaccumulation of fluid in the left upper quadrant compatible with a leak. Upper gastrointestinal endoscopy was again performed which showed a staple line defect in the gastric body superior to the previous defect. The defect was again closed with argon plasma ablation and an over-the-endoscope clip. However, UGIS post intervention revealed a persistent leak which prompted an exploratory laparotomy and drainage of the abscess. During the procedure an upper gastrointestinal endoscopy with underwater seal testing demonstrated no leaks and a Jackson Pratt drain was placed in the area. The patient was stable post-operatively and discharged 1 week later on a bariatric diet as tolerated and drain remaining in place. Four days after discharge, the patient returned with a 2-day history of epigastric pain and inability to tolerate oral intake. Complete blood count revealed a WBC count of 15,900 cells/mm3, and CT scan revealed a 5 × 1.4 × 2 cm collection in the left upper quadrant suspicious for an abscess for which the patient was treated with intravenous (IV) antibiotics. UGIS was done and revealed a leak proximal to the previous clips. The patient was managed with IV antibiotics and endoscopic placement of a 23 mm × 15.5 cm WallFlex covered stent which was sutured in place. The subphrenic collection was not drained and the patient was discharged 5 days later.
The patient presented to the emergency department 2 weeks later with progressively worsening shortness of breath and a cough for 1 week. The cough was productive of brown, foul-smelling sputum. The patient also reported pleuritic chest pain, fever, chills and decreased appetite associated with nausea but no vomiting. On examination, the patient was afebrile, blood pressure was 97/63 mm Hg, heart rate was 135 beats/min, respiratory rate was 22/min and oxygen saturation was 98% on room air. On examination, there was dullness to percussion of her chest and decreased breath sounds on the left lung base with the presence of bronchial breath sounds. Labs results showed a WBC count of 27,500 cells/mm3, and a computerized tomography angiography (CTA) (Fig. 1) on admission revealed a 10 cm intrapulmonary left lower lobe air and complex fluid collection, compatible with an intrapulmonary abscess. A follow-up CT scan with IV and enteral contrast (Fig. 2) of her abdomen and pelvis revealed a fistulous connection between the left upper abdomen and left chest cavity.
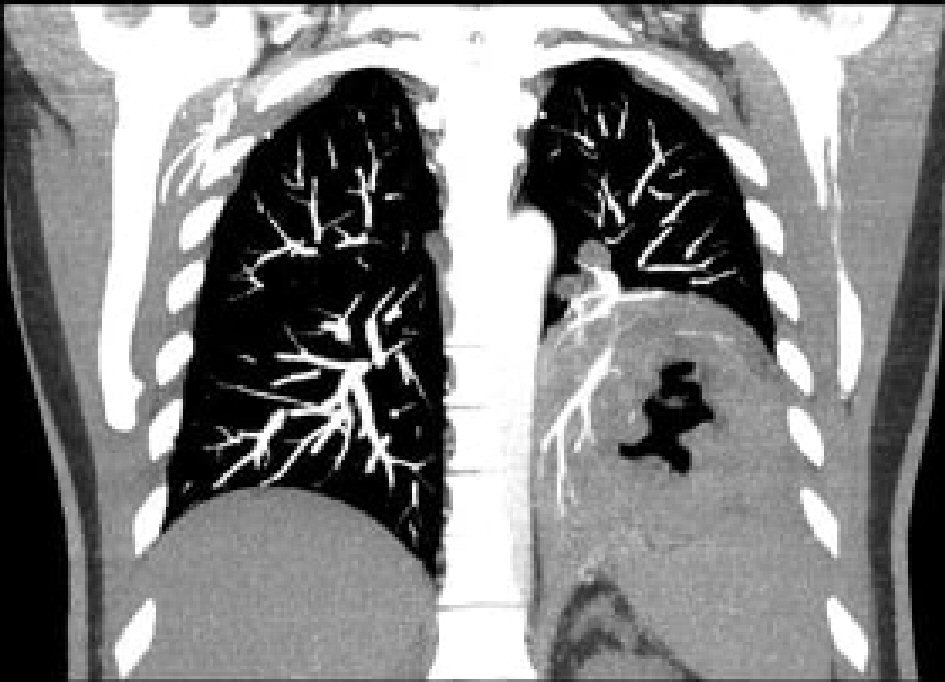 Click for large image | Figure 1. CTA revealing an intrapulmonary abscess in the left lower lung. CTA: computerized tomography angiography. |
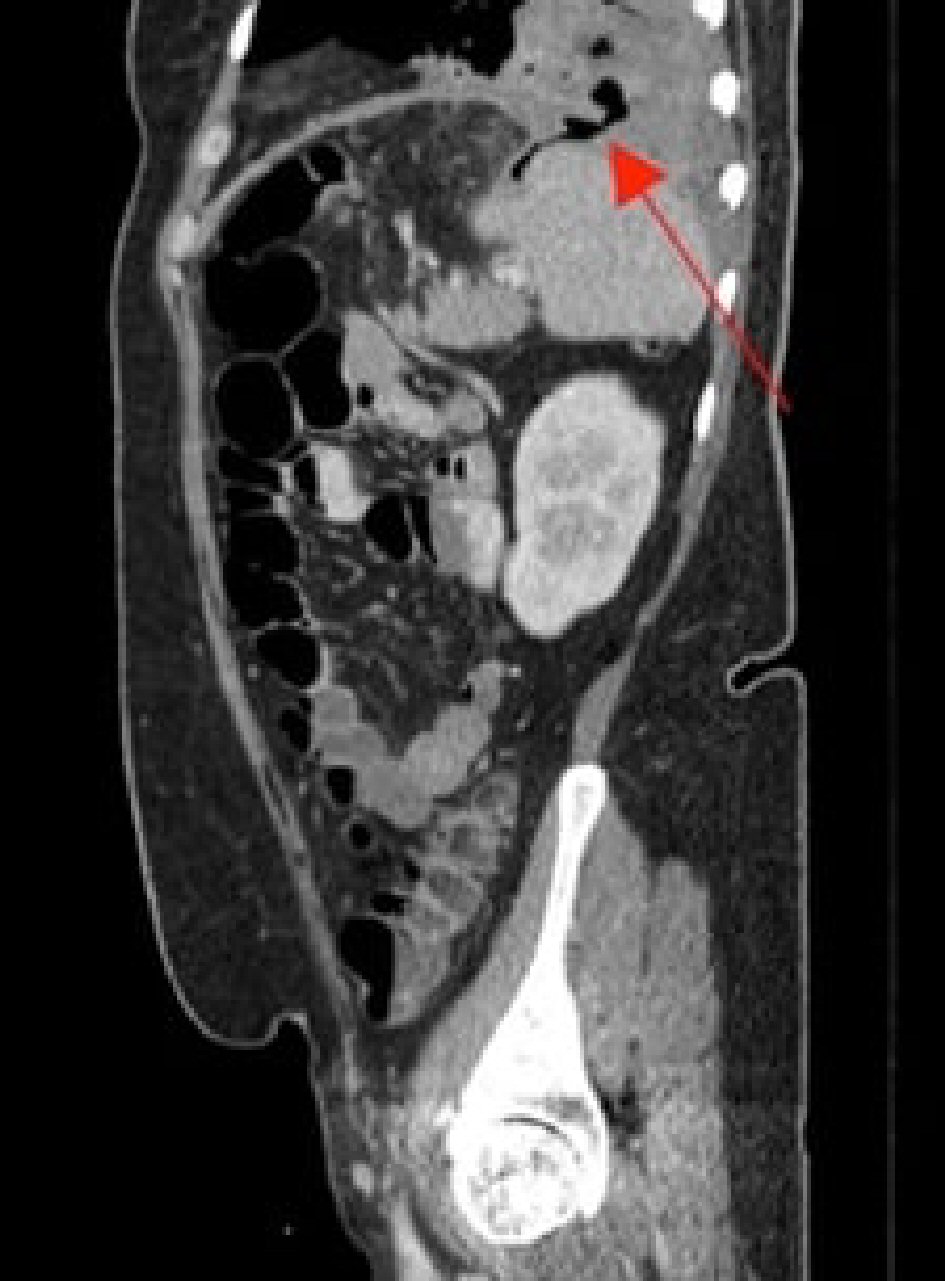 Click for large image | Figure 2. CT scan of the abdomen and pelvis showing a fistulous connection between the upper abdomen and left chest cavity (arrow). CT: computerized tomography. |
The patient was admitted to the surgical intensive care unit where she received 3 L of normal saline and was started on IV antibiotics. Once stable, it was determined that the patient required a left anterolateral thoracotomy for drainage of the left lower lobe abscess as it was too large to be drained percutaneously by interventional radiology. Intraoperatively, a large abscess was visualized in the left lower lobe with severe lower lobe distention. Purulent material was aspirated with a large bore catheter and the abscess was unroofed. The stent was then repositioned proximally and sutured in place to cover the localized area of the extravasation. A post-operative UGIS revealed no leak or obstruction. The patient continued to have an elevated WBC count and a repeat CT scan 10 days post-operatively revealed a 6 × 7.3 cm cavitary lesion in the left lower lobe communicating with the left pleural space. The patient underwent a second left thoracotomy 12 days after the initial operation. The accumulated purulent fluid was drained and a small wedge of the lower left lobe was resected. A repeat esophagogastroduodenoscopy (EGD) 4 days after the second thoracotomy revealed distal migration of the stent secondary to erosion of the recently placed suture. The stent was repositioned proximally and fixed in place with sutures. UGIS post intervention revealed no extravasation of contrast. The patient improved over the following week and was subsequently discharged.
| Discussion | ▴Top |
Obesity and its related complications have become a major public health issue. This has led to the development of surgical procedures that promote weight loss. LSG is a widely performed bariatric procedure due to its relative simplicity and comparable outcomes to the standard Roux-en-Y gastric bypass. LSG reduces the size of the stomach by 75-80% to improve satiety, improve insulin sensitivity and decrease fasting ghrelin levels [9]. Staple line leaks, staple line bleeding and strictures are complications of LSG which occur in up to 5.5%, 2% and 1% of cases, respectively [1-4]. Of these, staple line leakage is associated with the highest morbidity and mortality [1-5], and is described as acute (< 7 days), early (1 - 6 weeks), late (6 - 12 weeks) or chronic (> 12 weeks) [2, 4]. Factors that contribute to leaks include ischemia of the gastric wall near the staple line, and stenosis of the sleeve that leads to increased intraluminal pressure [1, 6]. About 85.7% of these leaks are found in the proximal one-third of the stomach near the gastroesophageal junction [2].
Early detection of gastric leaks is crucial to improve patient outcomes [2, 3]. A high index of clinical suspicion is of great importance in the detection of leaks as tachycardia, fever and abdominal pain are often the presenting signs [7]. This suspicion allows appropriate follow-up with imaging studies [5]. At the moment, there is no gold standard imaging study for diagnosing leaks. However, the most commonly performed studies include CT and UGIS. In a retrospective case series of 619 patients, 20 of whom had a gastric leak post bariatric surgery, CT with IV contrast was found to have a sensitivity of 95% and specificity of 100% for detecting gastric leaks, while UGIS had a sensitivity of 79% and specificity of 95% [5]. This may account for why our patient had a negative UGIS prior to discharge after LGS. However, it is also possible that our patient developed the initial leak after discharge.
Following the detection of leaks, adequate management is crucial to reduce recurrence and prevent the progression of leak-related complications. Management of gastric leaks are varied and should be based on the timing and clinical presentation [7]. Existing interventions include percutaneous, endoscopic and surgical approaches. Endoscopic procedures with percutaneous drainage are often used as they are minimally invasive and successful in 80-95% of cases [1, 2, 4, 7]. These procedures involve using self-expanding covered metal stents, clips, argon plasma ablation or glue injections. In cases with functional or mechanical obstruction, leaks may persist despite these interventions and progress to fistulas [2]. For the cases that develop fistulas, surgical intervention may be necessary.
Persistent gastric leaks may promote the formation of a GBF. GBF formation is a rare complication of LSG with unknown incidence due to the small number of reported cases in the literature [6]. In 1985, Moeller and Carpenter classified the causes of this condition into five categories: neoplasm, prior esophageal or gastric surgeries, trauma, gastric ulcers and subphrenic abscesses [9]. Of these, previous gastroesophageal surgery, gastric ulcers and subphrenic abscesses are the most commonly reported causes [8]. In the setting of a chronic gastric leak, the secretions can collect in the subphrenic region and result in a subphrenic abscess [8]. Due to its close proximity to the respiratory tract, abdominal infection can either spread by lymphatic flow or directly erode the diaphragm leading to a GBF. The progression of a chronic leak to a GBF may go undiagnosed as the subphrenic collection of gastric content can induce an omental “walling off” response that blocks and prevents diffuse peritonitis [6]. This may explain the delayed presentation of a GBF in our patient.
Immediate diagnosis and management of GBF is essential in reducing mortality from pulmonary infections, respiratory failure and septic shock. Since GBF is a rare complication of LSG, there are no well-established diagnostic and treatment algorithms [6]. UGIS and CT scan with oral contrast are acceptable imaging modalities used to diagnose GBF [6, 8], with CT scan having a higher sensitivity of 92% when fistulas were clinically suspected [2]. Measurement of pH of bronchial secretions may also be used to diagnose a GBF. However, it is only of diagnostic value in the absence of gastric aspiration [8, 10]. EGD provides minimal benefit in diagnosing GBF [8].
For many years, surgical intervention was considered the only way to treat GBF [3]. Surgical options described in literature include Roux-en-Y over the fistula, esophagojejunal anastomosis and total gastrectomy. However, this carries a significant risk of mortality ranging from 20% to 70%, most commonly due to aspiration pneumonia, acute respiratory distress syndrome and sepsis [2, 3, 6]. As a result, minimally invasive endoscopic procedures have been adapted to manage GBFs. A multicenter study of 15 patients who underwent endoscopic procedures reported a 93% success rate in closing GBFs. However, an average of 4.5 endoscopic sessions were required [2]. Endoscopic interventions included in this study were stricturotomies, dilations, gastric septoplasties and stents [2]. When stents were used, stent migration was the most common reported complication occurring in 15-60%, frequently requiring re-intervention to maintain coverage of the fistula [1, 2, 4, 11]. To minimize this, a report by Al-Lehibi described a successful case in which combined endoscopic approaches using over the scope clips and fully covered metal stents achieved successful healing of the fistula with no recurrence [3].
Conclusions
GBFs are a rare but life-threatening complication of LSG that can develop in the setting of a persistent gastric leak. This patient developed a subphrenic collection after presenting with recurrent staple line leaks. Unfortunately, this collection was not drained and likely resulted in the development of her GBF. This case report serves as an example of the importance of early diagnosis and management of suspected gastric leaks post-LSG
Acknowledgments
None to declare.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Ashen Fernando participated in patient care. Ashen Fernando and Jean Luc Francois wrote the case report. Nicole Majachani and Reshad Salam participated in the literature search. Stephanie Yee and Jamshed Zuberi revised and edited the case report.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Tsai YN, Wang HP, Huang CK, Chang PC, Lin IC, Tai CM. Endoluminal stenting for the management of leak following sleeve gastrectomy and loop duodenojejunal bypass with sleeve gastrectomy. Kaohsiung J Med Sci. 2018;34(1):43-48.
doi pubmed - Nguyen D, Dip F, Hendricks L, Lo Menzo E, Szomstein S, Rosenthal R. The surgical management of complex fistulas after sleeve gastrectomy. Obes Surg. 2016;26(2):245-250.
doi pubmed - Al-Lehibi A. Endoscopic management of gastrobronchial fistula after laparoscopic sleeve gastrectomy: a case report. Saudi J Med Med Sci. 2019;7(2):106-109.
doi pubmed - Guzaiz N, Arabi M, Khankan A, Salman R, Al-Toki M, Qazi S, Alzakari A, et al. Gastroesophageal stenting for the management of post sleeve gastrectomy leak. A single institution experience. Saudi Med J. 2016;37(12):1339-1343.
doi pubmed - Xu T, Rosculet N, Steele K, Auster M. Comparison of upper gastrointestinal fluoroscopy versus computed tomography for evaluation of post-operative leak in a bariatric surgery patient. BJR Case Rep. 2017;3(1):20160076.
doi pubmed - Ben Nun A, Simansky D, Rokah M, Zeitlin N, Golan N, Abu Khalil R, Soudack M. Surgical Treatment of Gastro-Pulmonary Fistula Following Bariatric Surgery: Possible and Safe. World J Surg. 2018;42(6):1792-1797.
doi pubmed - Sakran N, Goitein D, Raziel A, Keidar A, Beglaibter N, Grinbaum R, Matter I, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27(1):240-245.
doi pubmed - Jha PK, Deiraniya AK, Keeling-Roberts CS, Das SR. Gastrobronchial fistula—a recent series. Interact Cardiovasc Thorac Surg. 2003;2(1):6-8.
doi - Aryaie AH, Singer JL, Fayezizadeh M, Lash J, Marks JM. Efficacy of endoscopic management of leak after foregut surgery with endoscopic covered self-expanding metal stents (SEMS). Surg Endosc. 2017;31(2):612-617.
doi pubmed - Joseph JT, Krumpe PE. Diagnosis of gastrobronchial fistula by measurement of bronchial secretion pH. Case report and literature review. Chest. 1989;96(4):935-936.
doi pubmed - Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK. Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg. 2008;247(3):401-407.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
