| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 12, Number 1, March 2022, pages 21-23
Juvenile Atypical Ductal Hyperplasia: A Case Report
Sindi Dikoa, b, Lisa O’Kanea, Nadra Moulayesa
aDepartment of Surgery, St. Joseph’s University Medical Center, Paterson, NJ, USA
bCorresponding Author: Sindi Diko, Department of General Surgery, St. Joseph’s University Medical Center, Paterson, NJ 07503, USA
Manuscript submitted February 13, 2022, accepted March 2, 2022, published online March 12, 2022
Short title: A Case of Juvenile ADH
doi: https://doi.org/10.14740/jcs453
| Abstract | ▴Top |
Breast masses, benign and malignant, are extremely common in women. Benign breast masses have a variety of risk factors and are usually most common in women aged 30 - 40 years. Although much research has been conducted on benign breast disease and breast cancer in adult women, there remains a paucity of data on breast masses in adolescent women. More specifically, there is very little evidence regarding atypical ductal hyperplasia (ADH) in young women and the future risks it may carry as it is known to have a 4 to 5-fold increase for breast cancer in the adult population. We present a case of an 18-year-old female with ADH involving a fibroadenoma, with the hopes of highlighting the unique concerns and questions this diagnosis may bring to the young female population. Our patient’s questions centered around the risk this diagnosis carries of future cancer and what steps should be taken to minimize that risk. Although many models and guidelines exist to answer these questions in the older female, there is no consensus about treatment and monitoring ADH in a juvenile. By reviewing this case, we emphasize the need for future studies to quantify the risk of cancer progression from ADH in young females. We also demonstrate the need for guidelines to monitor and treat these findings in our younger populations.
Keywords: Atypical ductal hyperplasia; Juvenile; Adolescent
| Introduction | ▴Top |
Atypical ductal hyperplasia (ADH), a term given to a histological finding of breast tissue resembling low nuclear grade ductal carcinoma in situ (DCIS), is a rare finding in adolescent and young adult women [1]. ADH is considered to be a non-obligate precursor to ductal carcinoma with a 4 to 5-fold increased risk of developing breast cancer in bilateral breasts within 5 years [2] and up to 30% in 25 years [3, 4]. Whether this applies to adolescent and young women, however, has not been investigated. Since 1992, when juvenile ADH was first described, few additional case reports have been published, and even less research has been conducted to demonstrate what this means for this patient population [5-7]. Here, we present the case of an adolescent with ADH involving a fibroadenoma and review the current literature regarding ADH.
| Case Report | ▴Top |
Investigations
An 18-year-old healthy Asian female was referred by her primary care physician for evaluation of a right breast mass. She had no family history of breast cancer, denied any skin changes over the mass and had no nipple drainage or breast pain associated with the mass. On exam, she had an approximately 3-cm nodule at the retroareolar area mostly palpable between 6 - 8 o’clock. No skin dimpling, nipple inversion or nipple discharge was present, and no lymphadenopathy was appreciated.
Diagnosis
Our patient underwent a targeted ultrasound showing a 3.2 × 1.9 × 3.1 cm benign-appearing palpable mass in the right retroareolar region. Although this had a typical fibroadenoma appearance, given the size, the decision was made with the patient to remove the mass. Our differential included benign fibroadenoma, phyllodes tumor, or malignant mass.
Treatment
Shortly after, she underwent a right breast excisional biopsy. Two masses within the same specimen measuring at 1.2 × 1.0 × 1.0 cm and 3.8 × 3.0 × 2.8 cm, respectively, were identified on pathology. Grossly, they were noted to be tan-pink to white with typical whorled appearance consistent with fibroadenoma. The two masses, however, differed in their histology. The larger specimen was noted to display micropapillary and cribriform architectural with estrogen receptor (ER)-positive and cytokeratin 5/6 (CK 5/6)-negative staining, consistent with ADH involving a fibroadenoma (Fig. 1a, b). There was focal ADH present beyond the boundaries of the fibroadenoma bordering on carcinoma in situ (Fig. 2). The second smaller mass was noted to be a juvenile fibroadenoma.
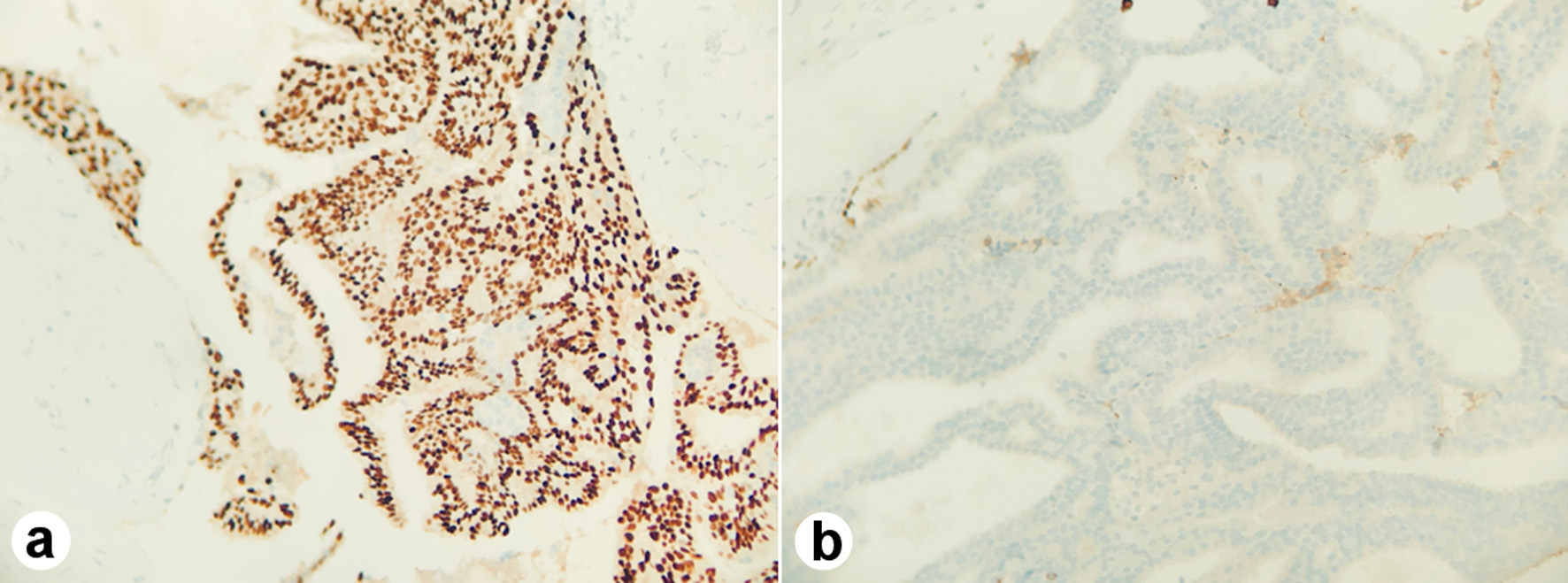 Click for large image | Figure 1. The micropapillary and cribriform architectural with estrogen receptor (ER)-positive (a) and cytokeratin 5/6 (CK 5/6)-negative staining (b). |
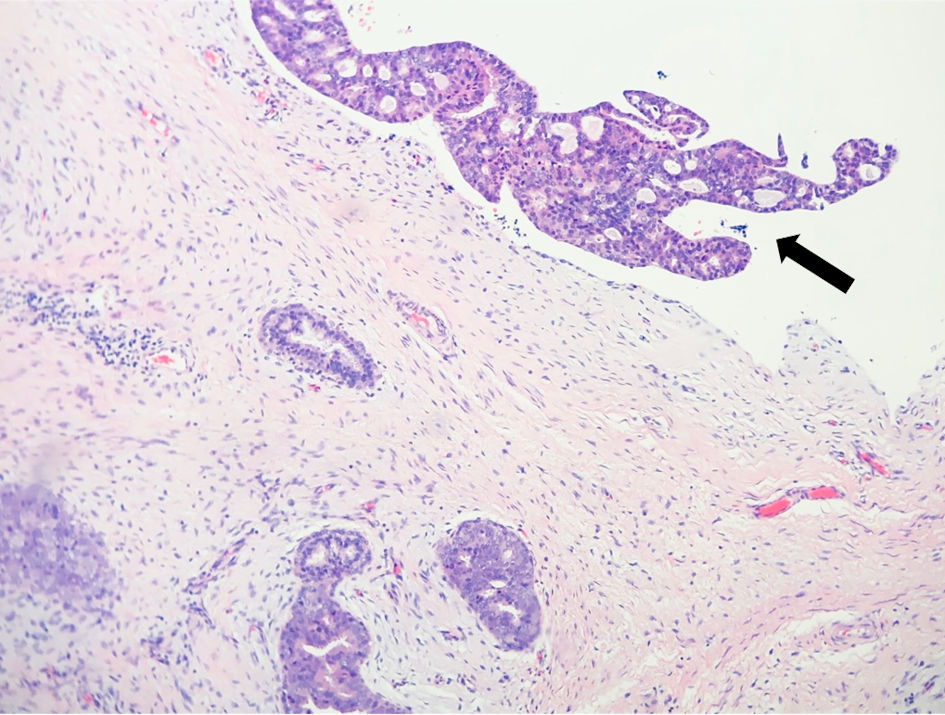 Click for large image | Figure 2. Atypical ductal hyperplasia (arrow) beyond the boundaries of the fibroadenoma. |
Follow-up and outcomes
Post operatively, she did well and healed appropriately. We recommended yearly annual exams and breast ultrasounds given that she did have atypia.
| Discussion | ▴Top |
Breast masses in adolescent and young adult females are overwhelmingly benign with the incidence of breast cancer in women younger than 25 years old being 3.2 per million [8]. The most common mass found in young women, especially women younger than 20, is a fibroadenoma. A juvenile or cellular fibroadenoma is a subset of adenomas characterized by hypercellular stromal proliferation and more rapid growth than a fibroadenoma with rare malignant transformations [9]. There is no increased cancer risk associated with fibroadenomas and management includes surgical excision only when the masses are symptomatic or rapidly growing [10].
Following biopsy based on atypical mammographic findings or a palpable breast mass, if ADH is found, there is a known cumulative risk of breast cancer of approximately 1% per year [11]. If diagnosed on core needle biopsy, excisional biopsy is recommended as it has been found to be upstaged to DCIS or carcinoma in 15-30% of cases [12]. Risk assessment models have been used to estimate future risk of breast cancer, including the Breast Cancer Risk Assessment Tool (BCRAT), a modified version of the well-known Gail model, and the International Breast Cancer Intervention Study (IBIS), also known as the Tyler-Cuzick model. Both have an adjustment for atypical hyperplasia, however the BCRAT has not been validated for women younger than 35 and shown to underestimate the risk of ADH, while the IBIS has been shown to overestimate the risk [13, 14].
Screening guidelines for women with high-risk lesions are offered by the National Comprehensive Cancer Network (NCCN), the American Cancer Society (ACS), and the American College of Radiology. However, all three indicate there is insufficient evidence to make recommendation for or against magnetic resonance imaging (MRI) screening in women with atypical hyperplasia [11]. Two studies to date have evaluated the role of MRI for ADH and have both concluded that it is not indicated [15, 16].
In regard to chemoprevention, the American Society of Clinical Oncology recommends chemoprevention with tamoxifen for premenopausal women or raloxifene in postmenopausal women with a 5-year absolute risk of breast cancer of 1.7% or greater [17]. Patients with ADH meet this criterion; however, it is unclear how often chemoprevention is used, and we are unable to find discussions regarding its use for juvenile ADH [11].
The most current guidelines regarding ADH are controversial and do not discuss best practices as it relates to juvenile ADH. Our patient highlights the need for further research into ADH, specifically within the adolescent and young adult age group in order to better guide her management.
Learning points
The incidence of ADH in young females remains widely uninvestigated and unknown. The current risk assessment models do not incorporate young age into their risk stratification which hinders accurate recommendations and treatment plans for young women with ADH. Considering these current circumstances, honest and open discussions with patients remain paramount. It is extremely important to present all the available information to make treatment and follow-up decisions as a care team.
Acknowledgments
We would like to thank the patient for allowing us to share her story.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
SD and LOK contributed to literature search, writing and editing. NM contributed to editing of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ADH: atypical ductal hyperplasia; DCIS: ductal carcinoma in situ; ER: estrogen receptor; CK 5/6: cytokeratin 5/6; BCRAT: Breast Cancer Risk Assessment Tool; IBIS: International Breast Cancer Intervention Study; NCCN: National Comprehensive Cancer Network; ACS: American Cancer Society
| References | ▴Top |
- Kader T, Hill P, Rakha EA, Campbell IG, Gorringe KL. Atypical ductal hyperplasia: update on diagnosis, management, and molecular landscape. Breast Cancer Res. 2018;20(1):39.
doi pubmed - Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146-151.
doi pubmed - Degnim AC, Visscher DW, Berman HK, Frost MH, Sellers TA, Vierkant RA, Maloney SD, et al. Stratification of breast cancer risk in women with atypia: a Mayo cohort study. J Clin Oncol. 2007;25(19):2671-2677.
doi pubmed - Hartmann LC, Radisky DC, Frost MH, Santen RJ, Vierkant RA, Benetti LL, Tarabishy Y, et al. Understanding the premalignant potential of atypical hyperplasia through its natural history: a longitudinal cohort study. Cancer Prev Res (Phila). 2014;7(2):211-217.
doi pubmed - Gandhi S, Sudheendra P, Rafferty W, Loveland-Jones C. Case report and review of adolescent atypical ductal hyperplasia and juvenile fibroadenoma: how do we assess and manage future risk? Clin Breast Cancer. 2018;18(5):e751-e754.
doi pubmed - Maroney J, et al. Incidental pathologic findings in adolescent and young adult reduction mammaplasty. Plastic and Reconstructive Surgery Global Open. 2019;7(8 Suppl):28-29.
doi - Eliasen CA, Cranor ML, Rosen PP. Atypical duct hyperplasia of the breast in young females. Am J Surg Pathol. 1992;16(3):246-251.
doi pubmed - Simmons PS, Jayasinghe YL, Wold LE, Melton LJ, 3rd. Breast carcinoma in young women. Obstet Gynecol. 2011;118(3):529-536.
doi pubmed - Chung EM, Cube R, Hall GJ, Gonzalez C, Stocker JT, Glassman LM. From the archives of the AFIP: breast masses in children and adolescents: radiologic-pathologic correlation. Radiographics. 2009;29(3):907-931.
doi pubmed - West KW, Rescorla FJ, Scherer LR, 3rd, Grosfeld JL. Diagnosis and treatment of symptomatic breast masses in the pediatric population. J Pediatr Surg. 1995;30(2):182-186; discussion 186-187.
doi - Hartmann LC, Degnim AC, Santen RJ, Dupont WD, Ghosh K. Atypical hyperplasia of the breast—risk assessment and management options. N Engl J Med. 2015;372(1):78-89.
doi pubmed - Kohr JR, Eby PR, Allison KH, DeMartini WB, Gutierrez RL, Peacock S, Lehman CD. Risk of upgrade of atypical ductal hyperplasia after stereotactic breast biopsy: effects of number of foci and complete removal of calcifications. Radiology. 2010;255(3):723-730.
doi pubmed - Pankratz VS, Hartmann LC, Degnim AC, Vierkant RA, Ghosh K, Vachon CM, Frost MH, et al. Assessment of the accuracy of the Gail model in women with atypical hyperplasia. J Clin Oncol. 2008;26(33):5374-5379.
doi pubmed - Boughey JC, Hartmann LC, Anderson SS, Degnim AC, Vierkant RA, Reynolds CA, Frost MH, et al. Evaluation of the Tyrer-Cuzick (International Breast Cancer Intervention Study) model for breast cancer risk prediction in women with atypical hyperplasia. J Clin Oncol. 2010;28(22):3591-3596.
doi pubmed - Port ER, Park A, Borgen PI, Morris E, Montgomery LL. Results of MRI screening for breast cancer in high-risk patients with LCIS and atypical hyperplasia. Ann Surg Oncol. 2007;14(3):1051-1057.
doi pubmed - Schwartz T, Cyr A, Margenthaler J. Screening breast magnetic resonance imaging in women with atypia or lobular carcinoma in situ. J Surg Res. 2015;193(2):519-522.
doi pubmed - Visvanathan K, Hurley P, Bantug E, Brown P, Col NF, Cuzick J, Davidson NE, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942-2962.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
