| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website https://www.currentsurgery.org |
Case Report
Volume 14, Number 1, May 2024, pages 11-16
Necrotizing Breast Infection: A Rare Entity and a Management Challenge
Hassan Masoudpoura, Jessica Wassefa, Alain Cagaananb, Jausheng Tzengb, Jenna Gillena, Rachelle Leonga, c
aDepartment of Surgery, Breast Surgical Services, Englewood Health, Englewood, NJ, USA
bDepartment of Pathology, Englewood Health, Englewood, NJ, USA
cCorresponding Author: Rachelle Leong, Department of Surgery, Breast Surgical Services, Englewood Health, Englewood, NJ 07631, USA
Manuscript submitted November 13, 2023, accepted November 27, 2023, published online April 1, 2024
Short title: A Case of Necrotizing Breast Infection
doi: https://doi.org/10.14740/jcs474
| Abstract | ▴Top |
Necrotizing infection of the breast associated with pre-existing malignancy, is a rarely encountered clinical scenario. This rarity, coupled with its potential life-threatening consequences and the complexities introduced by treatments like neoadjuvant chemotherapy and immunotherapy, highlights the need for timely and accurate intervention. We present the case of a 51-year-old female who was diagnosed with inflammatory triple-negative breast cancer. After undergoing neoadjuvant chemotherapy and immunotherapy, she developed a necrotizing infection of the breast. This required emergency surgical debridement and palliative mastectomy to remove the source of infection. This case underscores the crucial role of surgical management in such complex scenarios, particularly in the context of sepsis.
Keywords: Necrotizing infection; Triple-negative breast cancer; Inflammatory breast cancer; Surgical debridement; Sepsis; Palliative mastectomy
| Introduction | ▴Top |
Necrotizing breast infection is a rare, yet serious clinical condition often associated with pre-existing malignancies [1-3]. Its rarity makes early and accurate diagnosis challenging due to the wide differential of breast conditions including cellulitis, breast abscess, mastitis, masses, and inflammatory breast cancer [1, 3]. Delays in care can lead to life-threatening complications, including sepsis [1, 3, 4]. The disease process can be further complicated in patients undergoing treatments such as neoadjuvant chemotherapy and immunotherapy, as these therapies can potentially compromise the immune system, making the body more susceptible to infections [4]. Surgical management, including emergency surgical debridement or palliative mastectomy, is key, especially in severe cases with sepsis. Despite case complexity, effective surgical intervention can significantly enhance patient outcomes [1, 3].
| Case Report | ▴Top |
Investigations
A 51-year-old woman with a diagnosis of inflammatory, triple-negative right breast cancer presented to our institution. She has a family history of ovarian cancer with her mother at age 48. She has three children and denies a history of smoking or oral contraceptives. She reported initially noticing a red boil lesion and mass in her right breast 10 months prior to admission, which had acutely grown. A bilateral mammogram performed 3 months later at the time of visit revealed a 15 × 14 × 16 cm mass replacing the central and upper outer quadrant of the right breast. There were at least six enlarged axillary/axillary tail lymph nodes, with the largest matted lymph node group measuring 5 cm, and diffuse skin thickening observed. No abnormality was seen in the left breast. A stereotactic biopsy of the mass and axillary node was performed on the same day, revealing invasive ductal carcinoma, triple-negative, with a Ki-67 of 90%. The axillary node, however, was found to be benign. Staging computed tomography (CT) scan of chest, abdomen and pelvis showed right breast heterogenous mass, with central necrosis and multiple enlarged axillary lymphadenopathies involving level 2. An incidental right kidney lower pole mass was found, measuring up to 6.4 cm. No evidence of distant metastasis was reported.
Genetic testing was performed, and the results revealed no mutations. Neoadjuvant chemotherapy with Cytoxan-Adriamycin and taxane regimen was initiated. Despite undergoing this treatment, the patient’s condition did not show any improvement. Immunotherapy with pembrolizumab was added to the regimen. A positron emission tomography (PET) scan conducted 4 months later showed an ulcerated malignant neoplasm in the right breast, measuring up to 13 cm with heterogeneous tracer uptake (Fig. 1). The scan also revealed tracer avid skin thickening and subcutaneous fat induration, indicative of infiltrative malignancy and inflammation/infection. Metastatic disease was likely due to the presence of numerous tracer avid lymph nodes in the right axillary and subpectoral regions. The kidney mass standardized uptake value (SUV) was 3.8, likely a primary renal neoplasm.
 Click for large image | Figure 1. PET scan at 4 months revealing increased tracer uptake in the right breast. PET: positron emission tomography. |
Diagnosis
She presented to the emergency room 7 months after diagnosis with worsening weakness, vomiting and diarrhea without fever and chills. Upon arrival, she was hemodynamically stable but tachycardic. Her vital signs were recorded as follows: a blood pressure of 136/83 mm Hg, a heart rate of 109, a respiratory rate of 20, and a temperature of 36.3 °C. Key laboratory data included a white blood cell count of 10.87 × 103/µL, platelets at 44 × 103/µL, hemoglobin at 7.3 g/dL, and a low sodium level of 127 mmol/L. Her bicarbonate level was also low at 13 mmol/L, while there were raised levels of creatinine at 1.58 mg/dL, blood urea nitrogen at 66 mg/dL, glucose at 245 mg/dL, and beta-hydroxybutyrate at 0.72 mmol/L. Her glycated hemoglobin (HbA1c) was found to be 5.7%.
During the physical examination, the patient appeared distressed with altered mental status and lethargy. Her right breast had a large fungating mass with significant purulent drainage, erythematous necrotic tissue with violaceous and black discoloration. This condition encompassed her entire breast, which was tender to palpation (Fig. 2).
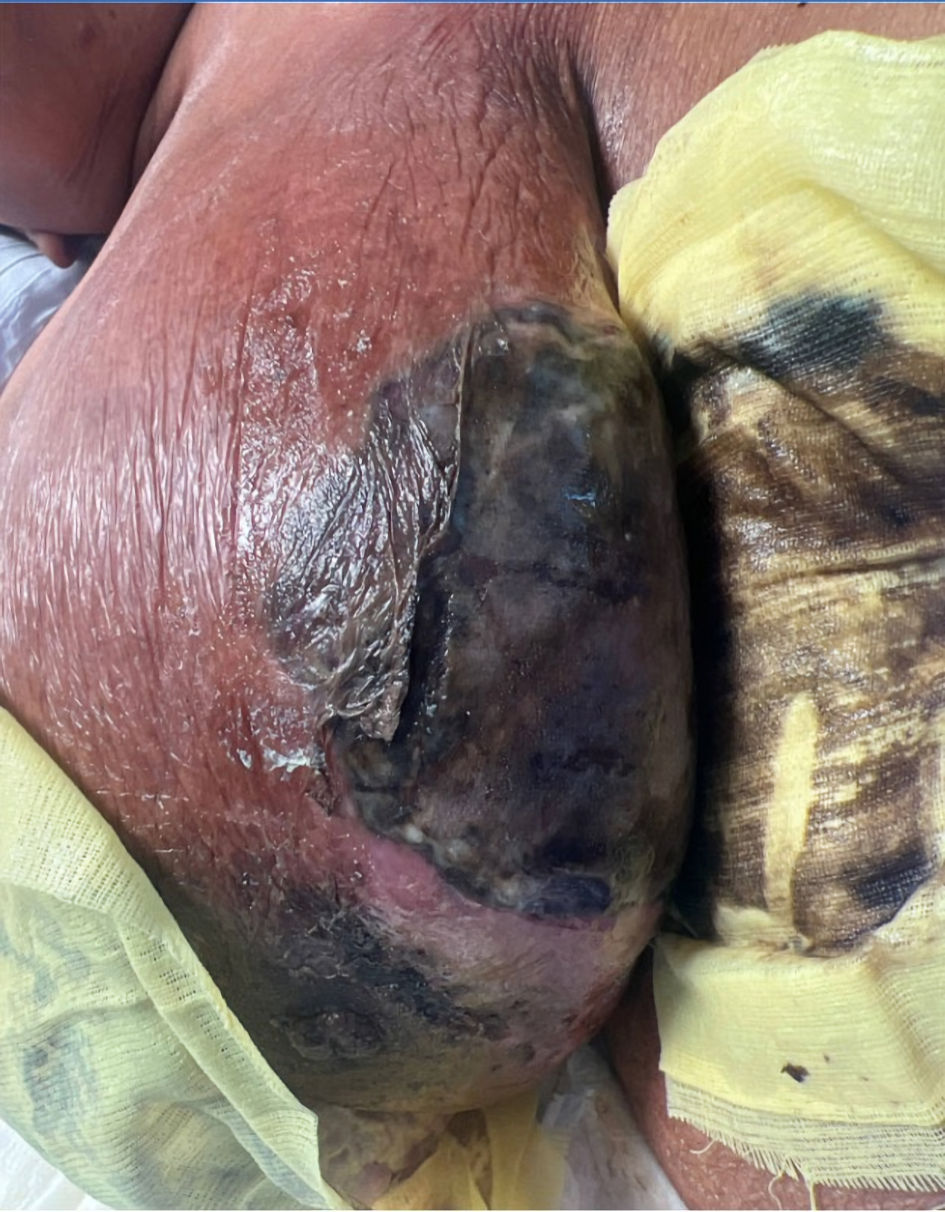 Click for large image | Figure 2. The image illustrating the condition of the breast upon arrival at the emergency room, characterized by intense erythema, skin de-epithelialization, ischemic changes, and tissue necrosis. |
Following admission, the patient received fluids for resuscitation, insulin sliding scale, and started on broad-spectrum intravenous antibiotics with meropenem and vancomycin. CT scan of the chest and abdomen pelvis showed signs of necrotizing fasciitis along with progression of her cancer with a new 4-mm right upper lobe nodule, hepatic metastasis, and left iliac bone metastasis (Fig. 3).
 Click for large image | Figure 3. CT scan of the chest and abdominopelvic of the breast necrotizing infection. This image illustrates large pockets of gas within the right breast parenchyma with edema. CT: computed tomography. |
Treatment
After multidisciplinary team discussion and careful consideration and discussion with the family, the decision was made to proceed with a palliative right breast mastectomy to remove the source of infection. She was transfused with one unit of platelets before being taken into the operating room for an urgent right total mastectomy.
An incision was made, revealing over 300 mL of purulent drainage with maggots within the tumor, drainage was sent for culture. The breast around the tumor and the suspicious area was excised, with the removed fascia from the muscle sent to pathology for examination (Fig. 4). Following excision, the wound was irrigated with saline, packed with Dakin’s-soaked dressing, and changed daily.
 Click for large image | Figure 4. Ulcerated and necrotic mass with cavitation involving breast tissue (gross pathology picture). |
Intraoperative cultures showed Bacteroides and Porphyromonas, Corynebacterium and Aactinomyces indicating type I necrotizing fasciitis. Pathology showed invasive ductal carcinoma, triple negative, measuring at least 16 cm with invasion to epidermis and extensive lymphatic permeation including involvement of dermal lymphatics. There was a large area of necrotizing inflammation with abscess formation, adjacent and extending from the tumor (Fig. 5).
 Click for large image | Figure 5. (a) Histologic features of invasive poorly differentiated ductal carcinoma. Sheets of atypical cells with areas of necrosis (original magnification × 100). (b) Scattered areas with squamous morules, compatible with an invasive ductal carcinoma with metaplastic squamous cell features (original magnification × 200). (c) Overlying skin with abundant dermal edema, acute inflammation, and tumor emboli in dermal lymphatic vessels (original magnification × 50). (d) Underlying fatty breast tissue with necrosis and abundant acute inflammation (original magnification × 10). |
Follow-up and outcomes
The patient was admitted to the intensive care unit for close monitoring, received two units of blood transfusion during their hospital stay, was discharged home with IV ertapenem treatment and wet-to-dry dressing with Dakin’s solution, and has an upcoming outpatient follow-up to examine the wound.
| Discussion | ▴Top |
Necrotizing breast infections are a rare and potentially life-threatening condition that may progress rapidly, presenting a highly complex clinical challenge that requires a multifaceted approach for early diagnosis and management [1, 3, 5].
It is crucial to understand the risk factors for early detection and early intervention of necrotizing fasciitis. Risk factors include individuals with compromised skin integrity due to trauma, biopsy, surgical procedures, chronic skin conditions such as pressure ulcers, liver disease, diabetes, cancer, immunocompromised patients with conditions such as acquired immunodeficiency syndrome (AIDS), transplant recipients, and those taking immunosuppressive or chemotherapy medications. They are inhibited from mounting an immune response characteristic of a healthier population, which can make diagnosis even more difficult and decompensation more rapid [3, 4, 6, 7].
The clinical manifestations of necrotizing breast infection closely resemble those observed in necrotizing infections in other body regions including pain out of proportion, skin changes such as erythema, necrosis, and crepitus. Patients may present with signs of sepsis and may have fever, altered mental status, elevated white blood cell count, or positive blood cultures complicated with septic shock and multiple organ failure [4, 8].
The greater challenge specific to breast is secondary to the wide differential unique to conditions of the breast that could potentially lead to a delay in diagnosis and diagnosis may be ambivalent. The differential may include cellulitis, breast abscess, mastitis, thrombophlebitis, or malignancy. Necrotizing fasciitis of the breast could also stem from these especially in cases of inflammatory breast cancer. Another challenge specific to breast is the delayed cutaneous involvement of necrotizing fasciitis in the breast secondary to the dense blood supply to the breast which may cause delayed diagnosis [3]. Therefore, it is important to frequently monitor patients with a high suspicion of necrotizing fasciitis as these infections can rapidly develop and the patient may develop septic shock after a short period.
Several biomarkers and scores, such as the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC), are used to differentiate necrotizing from non-necrotizing soft-tissue infections and predict patient outcomes. A LRINEC score of 6 or more differentiates necrotizing from non-necrotizing fasciitis [9].
In situations where the initial diagnosis is unclear, cross-sectional imaging like a CT scan could expedite the diagnosis process. However, it is crucial that imaging should not delay immediate surgical exploration, especially for patients clinically diagnosed with necrotizing infection as well as those in septic shock. Critical findings on CT scan that can indicate necrotizing fasciitis include low attenuation representing necrosis or abscess formation, with surrounding tissue enhancement and edema. Another typical feature is the presence of gas and fluid accumulation, as well as fascial involvement with asymmetrical fascial thickening [4, 8]. Magnetic resonance imaging (MRI) has also been described as a diagnostic tool and can outline the depth of the infection but has shown to have low specificity (46-86%) [10].
Another way to diagnose necrotizing fasciitis when uncertain is with an incisional biopsy of the area which can be performed at bedside. It is performed via a longitudinal incision or an incision over Langer’s lines. It is important to examine the underlying tissue for necrosis. Other classic signs upon incision include expression of dishwater pus, low resistance in the tissue and lack of bleeding, non-contracting muscle, and fascial edema [11]. Fascial biopsy material may be given to pathology in scenarios where it is still uncertain. Gram staining may reveal positive for group A Streptococcus (GAS) which can be performed rapidly. Cultures should also be obtained, but it is critical to obtain a tissue sample. The most specific finding is necrosis of the superficial fascia with perivasculitis and vasculitis along with fibrinous thrombi of arteries and vein located in the fascia. Polymorphonuclear may be visualized within the tissue [11].
The management of necrotizing breast infection involves a multi-faceted approach, requiring both broad-spectrum antibiotics and surgical intervention. The fundamental treatment strategy is early and aggressive surgical debridement of all necrotic tissue. Besides controlling the infection source, this approach facilitates the confirmation of necrotizing soft-tissue infection diagnosis, the acquisition of microbiological or histological samples, and the assessment of the scope of tissue necrosis [1, 4]. Palliative mastectomy aims to improve patient’s symptoms, optimize local infection control, improve overall well-being and quality of life, and potentially extend life expectancy [1, 12]. The majority of patients require a mastectomy for definitive source control. Partial mastectomy and radical mastectomies have also been performed and described for source control with a second look in 24 - 48 h for additional debridement [10, 12, 13]. All were initially closed by secondary intention or delayed primary closure. We previously reported a case of a breastfeeding woman who underwent an urgent mastectomy due to necrotizing fasciitis, followed by successful reconstruction with fasciocutaneous flaps; pathology findings revealed lactational changes, severe acute gangrenous inflammation, and abscesses consistent with acute necrotizing fasciitis. This case further underlines the importance of prompt diagnosis and immediate surgical intervention in managing necrotizing infections [1].
Reconstruction should be decided on a case-by-case basis. It depends on the amount of remaining breast tissue, size of the wound, infectious status, and patient comorbidities. A vacuum-assisted closure (VAC) device dressing can assist with increasing granulation of the wound and can further assist in closure of the wound. The most common method for closure was split thickness skin grafting, although a large proportion also undergo delayed primary closure [12, 13]. Delayed primary closure was a more likely option in those who underwent partial mastectomy with or without serial debridement. Hyperbaric oxygen therapy and use of the Integra bilayer wound matrix can help prepare the wound for future skin grafting [12-14]. Local flap advancement, local breast tissue arrangement, and pedicle flap reconstruction have also been described [12, 13].
Learning points
Necrotizing breast infection persists as a noteworthy and intricate facet with the spectrum of breast cancer care, especially in advanced scenarios. Timely recognition of the condition, the delineation of associated risk factors, and judicious choice of proper management strategies constitute pivotal determinants for optimizing patient prognosis.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
HM and JW conducted chart review, wrote the manuscript, conducted literature review, critically revised the manuscript, and provided final approval. AC and JT made a substantial contribution by providing pathological evaluation and pathology images. AC, JT, JG, and RL participated in manuscript writing, critically revised the manuscript, and final approval.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Gillen J, Verrico E, McIntosh V, Sussman B, Abramson D, Tzeng J. Case of necrotizing infection of the breast and brief review of literature. Breast J. 2020;26(5):1019-1021.
doi pubmed - Tariq J, Fatima K, Tariq MU, Zeeshan S. Necrotizing infection of the breast: a case report on a rare presentation of breast carcinoma. Cureus. 2022;14(4):e24504.
doi pubmed pmc - Fadaee N, Ong M, Al-Askari M. Necrotizing fasciitis of the breast: a case report and literature review. J Med Cases. 2019;10(9):288-292.
doi pubmed pmc - Hua C, Urbina T, Bosc R, Parks T, Sriskandan S, de Prost N, Chosidow O. Necrotising soft-tissue infections. Lancet Infect Dis. 2023;23(3):e81-e94.
doi pubmed - Golger A, Ching S, Goldsmith CH, Pennie RA, Bain JR. Mortality in patients with necrotizing fasciitis. Plast Reconstr Surg. 2007;119(6):1803-1807.
doi pubmed - Flandrin A, Rouleau C, Azar CC, Dubon O, Giacalone PL. First report of a necrotising fasciitis of the breast following a core needle biopsy. Breast J. 2009;15(2):199-201.
doi pubmed - Ward ND, Harris JW, Sloan DA. Necrotizing fasciitis of the breast requiring emergent radical mastectomy. Breast J. 2017;23(1):95-99.
doi pubmed - Marks B, Fasih T, Amonkar S, Pervaz M. Necrotising fasciitis of the breast: A rare but deadly disease. Int J Surg Case Rep. 2019;65:10-14.
doi pubmed pmc - Bechar J, Sepehripour S, Hardwicke J, Filobbos G. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: a systematic review of the literature. Ann R Coll Surg Engl. 2017;99(5):341-346.
doi pubmed pmc - Kaczynski J, Dillon M, Hilton J. Breast necrotising fasciitis managed by partial mastectomy. BMJ Case Rep. 2012;2012:bcr0220125816.
doi pubmed pmc - Hietbrink F, Bode LG, Riddez L, Leenen LP, van Dijk MR. Triple diagnostics for early detection of ambivalent necrotizing fasciitis. World J Emerg Surg. 2016;11:51.
doi pubmed pmc - Konik RD, Huang GS. Management of primary necrotizing fasciitis of the breast: a systematic review. Plast Surg (Oakv). 2020;28(4):215-221.
doi pubmed pmc - Islam S, Shah A, Maughn A, Dial S, Mahabir A, Naraynsingh V, Harnarayan P. Necrotizing soft tissue infections of the breast: a potentially lethal surgical emergency. Case Rep Surg. 2023;2023:4695019.
doi pubmed pmc - Marongiu F, Buggi F, Mingozzi M, Curcio A, Folli S. A rare case of primary necrotising fasciitis of the breast: combined use of hyperbaric oxygen and negative pressure wound therapy to conserve the breast. Review of literature. Int Wound J. 2017;14(2):349-354.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
