| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website http://www.currentsurgery.org |
Case Report
Volume 2, Number 4-5, October 2012, pages 149-153
Allergic Fungal Rhinosinusitis in the Lesser and Greater Wings of the Sphenoid With Resultant Optic Neuropathy: A Case Report and Review of the Literature
Mohamad R. Chaabana, c, Jayant M. Pintoa, Colin S. Poonb, Cheng Hongb, Jessie Awb
aThe University of Chicago Medical Center, Department of Surgery, Section of Otolaryngology, USA
bThe University of Chicago Medical Center, Department of Radiology, USA
cCorresponding author: Mohamad R Chaaban, 150 3rd Ave S. BDB 563, Birmingham, Al # 35205, USA
Manuscript accepted for publication August 8, 2012
Short title: Allergic Fungal Rhinosinusitis
doi: https://doi.org/10.4021/jcs93w
| Abstract | ▴Top |
The anatomy of the paranasal sinuses is important to be considered during clinical evaluation particularly for preoperative surgical planning. We report on a patient with allergic fungal sinusitis that presented with visual loss secondary to compressive optic neuropathy as a result of a rare anatomical variant of hyper-aerated lesser and greater wing of the sphenoid. This variant alone would not have come to medical attention were it not for the concomitant allergic fungal sinusitis causing expansion of the sinus cavities with resultant visual loss.
Keywords: Allergic fungal sinusitis; Fungal sinusitis; Chronic sinusitis; Chronic fungal sinusitis; Optic neuropathy
| Introduction | ▴Top |
Fungal sinusitis has increased in the immunocompetent population [1]. The American Academy of Otolaryngology-Head and Neck Surgery lists four types of fungal sinusitis: 1) mycetoma fungal sinusitis, 2) allergic fungal sinusitis which is considered the most common form, 3) chronic indolent sinusitis, and 4) fulminant sinusitis [2].
There are several well-defined anatomical variants for the sphenoid sinus; those related to the cavernous internal carotid artery, the vidian canal, the optic nerve, and the sphenoethmoid cell [3-5]. Hyper-aeration into the lesser and greater sphenoid wings is uncommon and typically asymptomatic. We report a rare case of extensive pneumatization of the greater and lesser wings of the sphenoid affected by allergic fungal sinusitis leading to compressive optic neuropathy.
| Case Report | ▴Top |
A thirty-one-year-old African-American male was referred by an outside ophthalmology clinic after being diagnosed with optic neuropathy secondary to sinusitis. He reported chronic blurry vision in his left eye, worsening over the past week. Examination in our office revealed decreased visual acuity in his left eye, loss of color vision and bilateral exophthalmia. Flexible nasopharyngoscopy showed massive edema of his nasal mucosa with purulent drainage bilaterally. It was impossible to view the middle turbinates on both sides except for their axillary attachments.
Imaging, which consisted of CT and MRI scans of the sinuses, was performed as part of our evaluation. The CT scan showed extensive pansinusitis with hyper-attenuation suggestive of fungal disease (Fig. 1). The sinuses appeared to be expanding into his orbits with hyper-pneumatization of the left greater and lesser wings of the sphenoid sinus extending to the lateral wall of the orbit resulting in exophthalmos (Fig. 2, 3). Magnetic resonance imaging (MRI) evaluation showed no extension into the central nervous system, and demonstrated findings consistent with narrowing of the left orbital apex and impingement of the left optic nerve (Fig. 3).
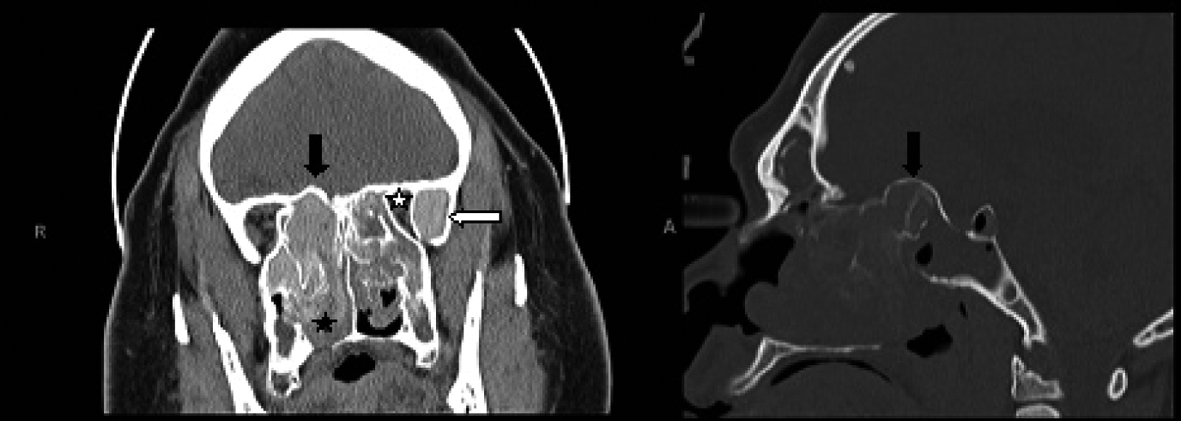 Click for large image | Figure 1. Coronal CT scan of the paranasal sinuses (left image) shows enlarged and opacified left greater wing of sphenoid with high attenuation material (white arrow), along with narrowing of the orbital apex on the left compared to the right side (white star). The fungal hyperattenuation areas also extend intranasally (black star). There is bulging of the roof of the posterior ethmoids into the intracranial space as demonstrated on both the coronal and sagittal CT scans (right image) labeled with black arrows. |
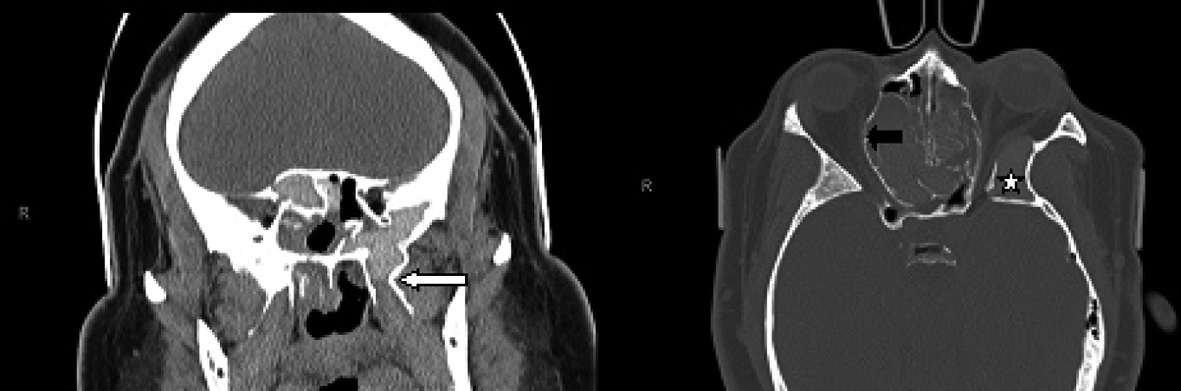 Click for large image | Figure 2. Coronal CT scan of the paranasal sinuses (left image) demonstrates the hyperaerated left sphenoid sinus extending far lateral and inferior with opacification of the left pterygoid (white arrow). The axial CT scan (right image) demonstrates bulging of the lamina papyracea into the orbits, more so on the right side (black arrow). There is redemonstration of the aerated and opacified left greater wing of sphenoid (white star). |
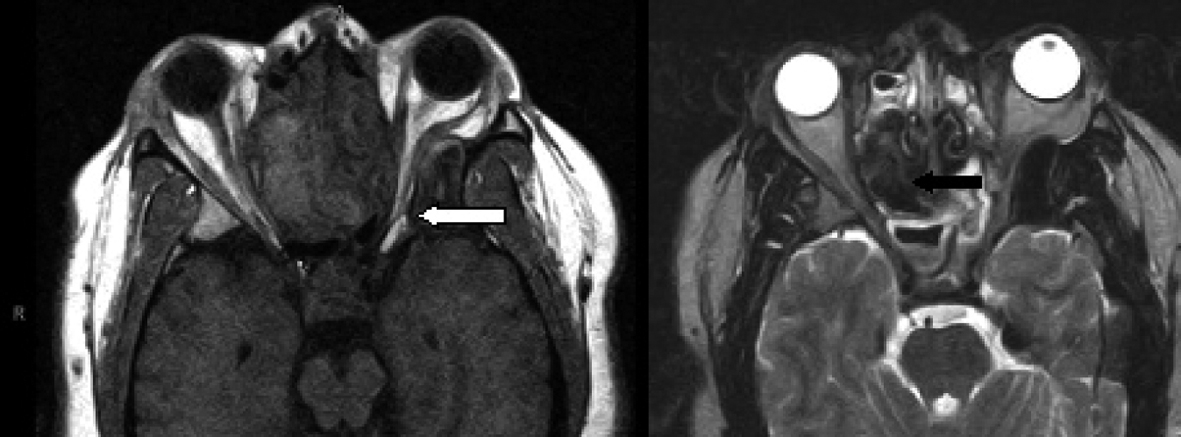 Click for large image | Figure 3. Axial MRI with T1-weighted (left image) and T2-weighted (right image) demonstrating the narrowing of the left orbital apex compared to the right side with impingement of the optic nerve (white arrow). Signal void is demonstrated with the black arrow on the T-2 weighted image. |
Due to the extensive disease, the patient ultimately underwent three staged bilateral endoscopic sinus surgeries addressing all four paranasal sinuses, with the last one including a left Caldwell-Luc procedure to remove persistent fungal debris and remnants of an extremely large infraorbital ethmoid cell. Aggressive irrigation of the sinuses during each operation was performed and intraoperative cultures were taken. The histology showed inflamed respiratory mucosa with numerous eosinophilia, reactive cancellous bone and methanamine/GMS stains that were positive for fungal hyphae, along with allergic mucin typical for allergic fungal sinusitis. The intraoperative sinus cultures grew Fusarium. Postoperatively the patient was placed on a course of oral and topical intranasal steroids as well as topical amphotericin B nasal irrigation. He regained his color vision, but had no improvement in visual fields. His recalcitrant disease is partly explained by the nature of his allergic fungal sinusitis, a disease whose etiology is controversial [6], but typically results in accumulation of fungal debris and production of allergic mucin with resultant severe chronic mucosal inflammation.
| Discussion | ▴Top |
Allergic fungal sinusitis is similar to the lower airway disorder known as allergic bronchopulmonary aspergillosis (ABPA) [7-9], but has been described as a distinct entity [10]. It occurs in immunocompetent hosts as a result of an inflammatory response to an offending but non-invasive mold [11], and is considered to be the most common type of fungal sinusitis in patients with chronic sinusitis [12]. The pathologic characteristics of allergic fungal sinusitis include fungal elements, absence of tissue invasion, and “allergic mucin” which is composed of cellular debris of broken down eosinophils and Charcot-Leyden crystals [13]. Diagnostic criteria were initially described by Bent and Kuhn and include: a type I hypersensitivity confirmed by histology, skin testing or serology; demonstration of fungi by histology or culture, presence of nasal polyposis, and hyperattenuation of debris in the affected sinus cavities on CT imaging [14]. The appearance of the sinuses on CT scan is dependent on the water content in addition to other features. If the sinus content is watery, then the attenuation on the CT scan is less than fat; however, as the secretions thicken, the attenuation will often exceed that of muscle [15, 16]. The hyperattenuation seen in the fungus are due to the calcium or magnesium salts deposited in the necrotic areas of the mycelia and mucin [17].
It is important to examine the appearance of the sphenoid sinus on CT scan. The normal physiological pneumatization of the sphenoid sinus leads to formation of recesses that are variably prominent; these include the opticocarotid recess, an aerated greater wing of the sphenoid, and the pterygoid recess. Given the close proximity to vital structures such as the pituitary, optic canal, carotid canal and the cavernous sinus, variations in its pneumatization can have important clinical implications as outlined in our case. To our knowledge there are few reports of complications from pneumatization into the greater and lesser wings of sphenoid [18]. In our case, both the lesser and greater wings of sphenoid bone were hyper-aerated and filled with fungal debris as detected intraoperatively. In addition to the findings on CT scan, MRI findings suggestive of allergic fungal sinusitis include low signal intensity on T1 weighted images and/or mixed signal intensities [19]. Such findings on MRI are likely due to fungus, but can also be present in a variety of pathologies such as in desiccated secretions, acute hemorrhage, calcium, bone and enamel [20].
A high index of suspicion of allergic fungal sinusitis is crucial for early diagnosis and proper treatment. Preoperative diagnosis will guide the surgeon on the surgical approach, extent of surgery and adjuvant medical therapy [21]. Although allergic fungal sinusitis usually follows a slow non-aggressive course, the disease can extend outside the confines of the sinuses with the possibility of massive bone destruction [22]. Bone erosion is more common in allergic fungal sinusitis than all the other types of inflammatory sinusitis combined [23] with studies reporting a higher incidence of bony erosions in males [23-27].
Reported ophthalmic manifestations of allergic fungal sinusitis include proptosis, diplopia, blepharoptosis, epiphora, opthalmoplegia, orbital abscesses and rarely visual loss [13, 28]. Visual loss associated with allergic fungal sinusitis is an uncommon finding (1.46% to 3.7 %) [29]. The pathophysiology of visual loss in patients with allergic fungal sinusitis has been proposed to be either through direct or indirect optic nerve compression, or through an inflammatory process that results in optic neuritis [29-33].
Compressive visual loss, which we believe is the culprit in our case, usually is a result of an initial venous occlusion with consequent tissue edema and nerve compression. This leads to a compartment syndrome and a vicious cycle of further edema and congestion ultimately leading to arterial compression and ischemic infarction of the nerve with complete visual loss [34]. This theory indicates that earlier venous congestion can lead to a reversible state of visual loss that can be reversed by decompression; however, arterial infarction is associated with complete visual loss and hence irreversibility.
Management of patients consists of medical and surgical treatment. ESS is needed in addition to adjuvant medical therapy in order to rid the sinuses of the fungal elements and debris [22, 24, 35]. "Medical decompression", has been advocated by some, and is accomplished by the administration of high dose systemic steroids [33]; however, this approach is usually performed before and after surgery to assist in the return of the inflamed mucosa to a normal state and minimize recurrence [36]. It has been reported that if surgical decompression is delayed 30 days or more from the onset of visual loss, patients will not have significant benefit from the surgery [37]. As far as adjuvant treatment, and in addition to topical and oral steroids, multiple studies have been conducted on the use of antifungals including intranasal amphotericin B. These studies were done on patients who had CRS [38-45] with only one study that excluded allergic fungal sinusitis [44]. Of all these studies, three of them were based on double blind, placebo-controlled studies [39, 41, 46]. These studies were conflicting regarding their treatment effect, with the largest multicenter, double blind controlled study showing negative result for the use of amphotericin B in CRS patients [41]. In our case, amphotericin B nasal irrigation was used as an adjunct treatment postoperatively in combination to oral and topical steroids. Unfortunately as indicated in our history, the patient had recurrence of his disease despite maximal medical management.
We believe that the mechanism of our patient’s visual loss is consistent with what was reported in the literature regarding compressive optic neuropathy in allergic fungal sinusitis patients [29]. To our knowledge, there are no reports in the literature describing such compression as caused by the hyper-aeration of the greater and lesser wings of the sphenoid. In addition, this sphenoid variant would not have come to medical attention were it not for the concomitant allergic fungal sinusitis causing expansion of the sinus cavities with resultant visual loss.
Conclusions
In summary, we report an unusual case of hyper aeration of the greater and lesser wings of the sphenoid associated with visual loss secondary to allergic fungal sinusitis. The surgeon and radiologist should be aware of common and uncommon variants in order to avoid misinterpretation and to avoid the potential morbidity from surgical intervention if misinterpretation has occurred. Awareness of the variants of rhinosinusitis is crucial in the care of rhinologic patients.
Financial Disclosures
No financial disclosures.
Conflicts of Interest
None.
| References | ▴Top |
- Hadi U, Bitar M, Hachem R, Saade R, Husni R, Raad I. Fungal sinusitis in the immunocompetent patient: risk factors and surgical management. Surg Infect (Larchmt). 2003;4(2):199-204.
pubmed doi - Understanding fungal sinusitis, Fungal sinusitis Information; Sinus news 2006. www.sinusnews.com.
- Kantarci M, Karasen RM, Alper F, Onbas O, Okur A, Karaman A. Remarkable anatomic variations in paranasal sinus region and their clinical importance. Eur J Radiol. 2004;50(3):296-302.
pubmed doi - Sirikci A, Bayazit YA, Bayram M, Mumbuc S, Gungor K, Kanlikama M. Variations of sphenoid and related structures. Eur Radiol. 2000;10(5):844-848.
pubmed doi - Kazkayasi M, Karadeniz Y, Arikan OK. Anatomic variations of the sphenoid sinus on computed tomography. Rhinology. 2005;43(2):109-114.
pubmed - Schubert MS. Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol. 2009;47 Suppl 1:S324-330.
pubmed - Manning SC, Schaefer SD, Close LG, Vuitch F. Culture-positive allergic fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1991;117(2):174-178.
pubmed doi - Dolen WK. Risk factors for Allergic aspergillus sinusitis. Med Mycol 2006; 44: 273-275.
- Manning SC, Merkel M, Kriesel K, Vuitch F, Marple B. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope. 1997;107(2):170-176.
pubmed doi - Mukherji SK, Figueroa RE, Ginsberg LE, Zeifer BA, Marple BF, Alley JG, Cooper LL, et al. Allergic fungal sinusitis: CT findings. Radiology. 1998;207(2):417-422.
pubmed - Coop CA, England RW. Allergic fungal sinusitis presenting with proptosis and diplopia: a review of ophthalmologic complications and treatment. Allergy Asthma Proc. 2006;27(1):72-76.
pubmed - Klapper SR, Lee AG, Patrinely JR, Stewart M, Alford EL. Orbital involvement in allergic fungal sinusitis. Ophthalmology. 1997;104(12):2094-2100.
pubmed - Kirszrot J, Rubin PA. Invasive fungal infections of the orbit. Int Ophthalmol Clin. 2007;47(2):117-132.
pubmed doi - Bent JP, 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111(5):580-588.
pubmed doi - Yousem DM. Imaging of sinonasal inflammatory disease. Radiology. 1993;188(2):303-314.
pubmed - Som PM, Curtin HD. Chronic inflammatory sinonasal diseases including fungal infections. The role of imaging. Radiol Clin North Am. 1993;31(1):33-44.
pubmed - Kopp W, Fotter R, Steiner H, Beaufort F, Stammberger H. Aspergillosis of the paranasal sinuses. Radiology. 1985;156(3):715-716.
pubmed - Terra ER, Guedes FR, Manzi FR, Boscolo FN. Pneumatization of the sphenoid sinus. Dentomaxillofac Radiol. 2006;35(1):47-49.
pubmed doi - Aribandi M, McCoy VA, Bazan C, 3rd. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007;27(5):1283-1296.
pubmed doi - Som PM, Dillon WP, Curtin HD, Fullerton GD, Lidov M. Hypointense paranasal sinus foci: differential diagnosis with MR imaging and relation to CT findings. Radiology. 1990;176(3):777-781.
pubmed - Dhiwakar M, Thakar A, Bahadur S, Sarkar C, Banerji U, Handa KK, Chhabra SK. Preoperative diagnosis of allergic fungal sinusitis. Laryngoscope. 2003;113(4):688-694.
pubmed doi - Kinsella JB, Rassekh CH, Bradfield JL, Chaljub G, McNees SW, Gourley WK, Calhoun KH. Allergic fungal sinusitis with cranial base erosion. Head Neck. 1996;18(3):211-217.
pubmed doi - Ghegan MD, Lee FS, Schlosser RJ. Incidence of skull base and orbital erosion in allergic fungal rhinosinusitis (AFRS) and non-AFRS. Otolaryngol Head Neck Surg. 2006;134(4):592-595.
pubmed doi - Liu JK, Schaefer SD, Moscatello AL, Couldwell WT. Neurosurgical implications of allergic fungal sinusitis. J Neurosurg. 2004;100(5):883-890.
pubmed doi - Mian MY, Kamal SA, Senthilkumaran G, Abdullah A, Pirani M. Allergic fungal rhinosinusitis: current status. Pak J Otolaryngol 2002;18:36-40.
- Iqbal K, Saqlain G, Jalisi M. Nasal polyposis and fungal sinusitis -efficacy of Caldwell Luc as a therapeutic procedure. Pak J Otolaryngol 1993;9:173-6.
- Akhtar MR, Ishaque M, Saadat U. Aetiology of nasal polyp – a study of 200 cases at Combined Military Hospital Rawalpindi. Pak J Otolaryngol 2004;20:9-11.
- Carter KD, Graham SM, Carpenter KM. Ophthalmic manifestations of allergic fungal sinusitis. Am J Ophthalmol. 1999;127(2):189-195.
pubmed doi - Marple BF, Gibbs SR, Newcomer MT, Mabry RL. Allergic fungal sinusitis-induced visual loss. Am J Rhinol. 1999;13(3):191-195.
pubmed doi - Gupta AK, Bansal S, Gupta A, Mathur N. Visual loss in the setting of allergic fungal sinusitis: pathophysiology and outcome. J Laryngol Otol. 2007;121(11):1055-1059.
pubmed doi - Attallah M, Hashash M, al-Muhaimeed H, Dousary S, al Rabah A, Kharashi S. Reversible neuropraxic visual loss induced by allergic Aspergillus flavus sinomycosis. Am J Rhinol. 1999;13(4):295-298.
pubmed doi - Graham SM, Carter KD. Response of visual loss in allergic fungal sinusitis to oral corticosteroids. Ann Otol Rhinol Laryngol. 2005;114(3):247-249.
pubmed - Elwany S, Elsaeid I, Thabet H. Endoscopic anatomy of the sphenoid sinus. J Laryngol Otol. 1999;113(2):122-126.
pubmed doi - Chen YR, Breidahl A, Chang CN. Optic nerve decompression in fibrous dysplasia: indications, efficacy, and safety. Plast Reconstr Surg. 1997;99(1):22-30; discussion 31-23.
pubmed - Nussenbaum B, Marple BF, Schwade ND. Characteristics of bony erosion in allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2001;124(2):150-154.
pubmed doi - Ence BK, Gourley DS, Jorgensen NL. Allergic fungal sinusitis. Am J Rhinol 1990;4:169-78.
- Thakar A, Lal P, Dhiwakar M, Bahadur S. Optic nerve compression in allergic fungal sinusitis. J Laryngol Otol. 2011;125(4):381-385.
pubmed doi - Ponikau JU, Sherris DA, Kita H, Kern EB. Intranasal antifungal treatment in 51 patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2002;110(6):862-866.
pubmed doi - Ponikau JU, Sherris DA, Weaver A, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: a randomized, placebo-controlled, double-blind pilot trial. J Allergy Clin Immunol. 2005;115(1):125-131.
pubmed doi - Corradini C, Del Ninno M, Buonomo A, Nucera E, Paludetti G, Alonzi C, Sabato V, et al. Amphotericin B and lysine acetylsalicylate in the combined treatment of nasal polyposis associated with mycotic infection. J Investig Allergol Clin Immunol. 2006;16(3):188-193.
pubmed - Ebbens FA, Scadding GK, Badia L, Hellings PW, Jorissen M, Mullol J, Cardesin A, et al. Amphotericin B nasal lavages: not a solution for patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2006;118(5):1149-1156.
pubmed doi - Helbling A, Baumann A, Hanni C, Caversaccio M. Amphotericin B nasal spray has no effect on nasal polyps. J Laryngol Otol. 2006;120(12):1023-1025.
pubmed doi - Ricchetti A, Landis BN, Maffioli A, Giger R, Zeng C, Lacroix JS. Effect of anti-fungal nasal lavage with amphotericin B on nasal polyposis. J Laryngol Otol. 2002;116(4):261-263.
pubmed doi - Weschta M, Rimek D, Formanek M, Polzehl D, Podbielski A, Riechelmann H. Topical antifungal treatment of chronic rhinosinusitis with nasal polyps: a randomized, double-blind clinical trial. J Allergy Clin Immunol. 2004;113(6):1122-1128.
pubmed doi - Gerlinger I, Fittler A, Fonai F, Patzko A, Mayer A, Botz L. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis, with a review of the antifungal therapy. Eur Arch Otorhinolaryngol. 2009;266(6):847-855.
pubmed doi - Shin SH, Ye MK. Effects of topical amphotericin B on expression of cytokines in nasal polyps. Acta Otolaryngol. 2004;124(10):1174-1177.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
