| Journal of Current Surgery, ISSN 1927-1298 print, 1927-1301 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Curr Surg and Elmer Press Inc |
| Journal website http://www.currentsurgery.org |
Short Communication
Volume 3, Number 2, October 2013, pages 82-86
Pharmacokinetics of Ketamine and Norketamine After Oral Administration of a Liquid Formulation of Ketamine
Rie Kubotaa, d, Takako Komiyamaa, Yasuko Miwab, Seiko Buna, Junichiro Ishiic, Sadatsugu Mineic, Akira Iriec
aPharmacy Practice and Sciences, Department of Clinical Pharmacy, Center for Clinical Pharmacy and Sciences, School of Pharmacy, Kitasato University, 5-9-1 Shirokane, Minato-ku, Tokyo, 108-8641, Japan
bDepartment of Anaesthesia, National Institute of Cancer Hospital East, 6-5-1 Kashiwanoha, Kashiwa-shi, Chiba, 277-8577, Japan
cDepartment of Urology, Kitasato University Kitasato Institute Hospital, 5-9-1 Shirokane, Minato-ku, Tokyo, 108-8642, Japan
dCorresponding author: Rie Kubota, 5-9-1 Shirokane, Minato-ku, Tokyo, 108-8641, Japan
Manuscript accepted for publication July 25, 2013
Short title: Pharmacokinetics of Ketamine and Norketamine
doi: https://doi.org/10.4021/jcs196e
| Abstract | ▴Top |
Background: Ketamine and its active metabolite, norketamine, have analgesic actions. A liquid formulation of ketamine has been prepared in some hospital pharmacies. The pharmacokinetic parameters of ketamine and norketamine after oral administration have not yet been reported. The objective of this study was to evaluate the pharmacokinetics of oral liquid ketamine in healthy volunteers.
Methods: A liquid formulation of ketamine (1%) was administered to six healthy volunteers at a single oral dose of 5 mL. Venous blood samples (10 mL each) were collected before the administration, and at 0.25, 0.5, 0.75, 1, 1.5, 2, 6, 12 hours after the administration of ketamine. The blood pressure and pulse were measured, and the subjective symptoms were also checked for. The serum concentrations of ketamine and norketamine were measured by the HPLC method.
Results: The Cmax, Tmax and AUC0-12h of ketamine/norketamine were 29.9 ± 5.3/250.2 ± 28.7 ng/mL, 1.1 ± 0.2/1.6 ± 0.2 h, and 79.0 ± 24.0/1193.1 ± 159.6 ng·hr/mL, respectively. All of the six subjects reported feeling drunk temporarily around the Tmax of ketamine, and two of the six subjects complained of feeling sleepy around the Tmax of ketamine and norketamine. Furthermore, a significant increase of the mean systolic blood pressure was also noted at 1 and 1.5 hours after administration of ketamine.
Conclusions: Norketamine may contribute to the analgesic effect after oral administration of ketamine. Thus, the liquid formulation of ketamine may be a useful formulation for obtaining effective analgesia.
Keywords: Ketamine; Norketamine; Analgesia; Liquid formulation; Pharmacokinetics; Healthy volunteer; HPLC; Safety
| Introduction | ▴Top |
Ketamine is a dissociative anesthetic with analgesic and anesthetic properties. It is known to exert analgesic effects when administered at half its anesthetic dose, and been shown to be effective against neuropathic pain. At low doses, ketamine is a non-competitive NMDA receptor antagonist with analgesic effect [1, 2]. It has been suggested that ketamine and its active metabolite, norketamine, have analgesic properties. However, the pharmacokinetics of ketamine and norketamine have not yet been investigated following administration of ketamine by various routes for obtaining an analgesic effect [3-6].
Ketamine has been administered by the IV and IM routes for obtaining analgesia, as well in the tablet, capsule, liquid, suppository or nasal spray forms in hospital pharmacy settings [7-10]. There is no commercially available formulation of ketamine except for injection. Especially, a liquid formulation is prepared in some hospital pharmacies. However, the pharmacokinetic parameters of ketamine and norketamine after oral administration have not yet been reported [11-15]. In addition, the effects and safety of liquid formulations of ketamine have not been investigated well, and the optimal dosage of ketamine for obtaining an analgesic effect is not yet known.
In the present study, we investigated the pharmacokinetics of oral liquid ketamine in healthy volunteers.
| Materials and Methods | ▴Top |
This study was performed with the approval of the Kitasato Institute Hospital research committee, in compliance with the ethical principles laid out in the Declaration of Helsinki and all subjects signed a consent form for participation in the study.
Preparation of ketamine
A liquid formulation containing 10 mg per mL of ketamine was prepared, with the following formula: ketamine (Ketalar 50 mg/mL, Daiichi-Sankyo, Tokyo, Japan), simple syrup, and purified water (1:2:1); green apple flavor was then added because ketamine has a bitter taste.
Patients and drug administration
The subjects were six healthy volunteers (three male, three female), who had provided informed consent for participation in this study. The exclusion criteria were: presence of severe cardiovascular disease, hypertension, renal dysfunction or known hypersensitivity to ketamine.
All the subjects were administered 5 mL of the oral liquid formulation of ketamine (1%) after a meal. Venous blood samples (10 mL each) were collected before administration, and at 0.25, 0.5, 0.75, 1, 1.5, 2, 6, 12 hours after the administration of ketamine.
Blood pressure and pulse were measured at each sampling point, and subjective symptoms, including sleepiness and nausea, were also checked for. Some laboratory parameters, including serum AST, ALT, LDH, BUN and Cr were measured before and after the study.
Analysis of ketamine and norketamine
Ketamine and norketamine were measured by the HPLC method described by Sebastien [16], 1 mL of serum was alkalinized with 350 µL of 0.2 mol/L boric acid buffer (pH 13) and extracted into 5 mL dichloromethane:ethyl acetate (80:20 v/v%). After evaporation, the organic phase was added to 500 µL of dichloromethane:ethyl acetate (80:20 v/v%) and back-extracted into 2 mL of 2 mol/L HCl. The HCl phase was evaporated and the residue reconstituted in 200 µL of the mobile phase. The HPLC column used was PurospherSTR RP-18 endcapped (MERCK). The mobile phase consisted of acetonitrile and 0.03 mol/L K2HPO4(27:73 v/v pH 6.8). The flow rate was 1.5 mL/min. The detection wavelength was 210 nm.
Pharmacokinetic analysis
The serum concentration profiles of ketamine and norketamine were fitted using a nonlinear least-squares program, PSAG-CP (Asumedica, Osaka, Japan), and the parameters measured were the area under the concentration-time curve (AUC), the maximum concentration (Cmax), the time to reach Cmax (Tmax), T1/2, Cltot/F, and Vdss.
| Results | ▴Top |
The subjects were three male and three female healthy volunteers, ranging in age from 24.5 ± 1.6 years and having a body weight of 61.5 ± 11.0 kg. The serum concentration-time profiles for ketamine and norketamine measured over a period of 12 hours after administration of the liquid formulation of ketamine is shown in Figure 1. Ketamine and norketamine were detected in the serum starting at 0.25 hours after administration of ketamine. The Cmax, Tmax and AUC0-12h of ketamine and norketamine were 29.9 ± 5.3/250.2 ± 28.7 ng/mL, 1.1 ± 0.2/1.6 ± 0.2 h, and 79.0 ± 24.0/1193.1 ± 159.6 ng·hr/mL, respectively. The values of the other pahrmacokinetic parameters of ketamine and norketamine are shown in Table 1.
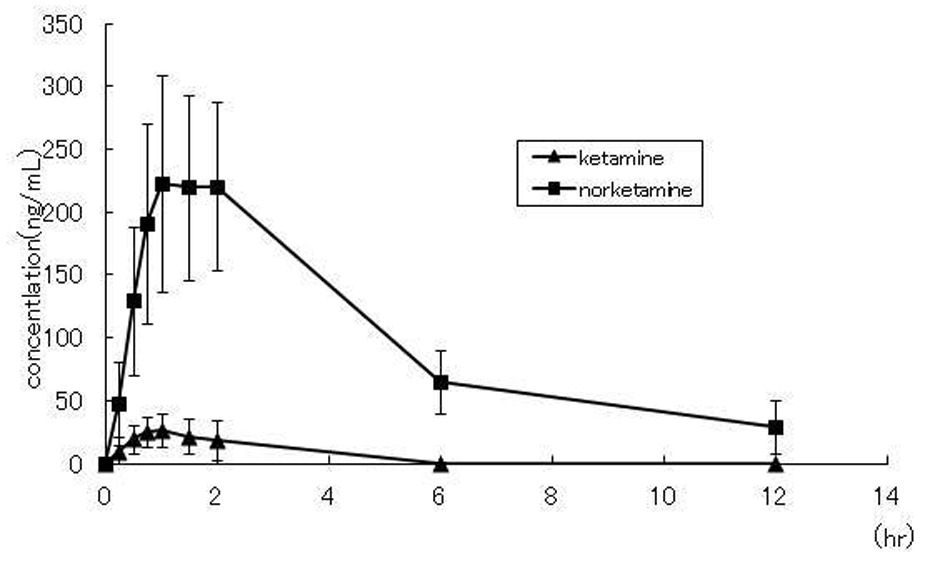 Click for large image | Figure 1. Concentration-time plots for ketamine and norketamine after oral administration of 50 mg ketamine to healthy volunteers (n = 6) |
 Click to view | Table 1. Pharmacokinetic Parameters of Ketamine and Norketamine |
The side effects after administration of ketamine until 12 hours are shown in Table 2. All of the six subjects felt drunk temporarily around the Tmax of ketamine, two of the six complained of feeling sleepy around the Tmax of ketamine and norketamine, and only one complained of slight nausea. In addition, a significant increase of the mean systolic blood pressure was observed at 1 and 1.5 hours after administration of ketamine. No changes of the renal or liver function parameters were observed after the drug administration as compared to the values recorded before the administration. No severe side effects were noted in any of the subjects.
 Click to view | Table 2. Adverse Reactions of Ketamine Observed Until 12 hours After Administration |
| Discussion | ▴Top |
In the present study, the pharmacokinetics and safety of a liquid formulation of ketamine administered at a single oral dose of 50 mg were determined in six healthy volunteers.
Persistent noxious stimuli activate the NMDA receptor, which is one of the excitatory amino acid receptors, in the posterior horn of the spinal cord, and lead to chronic pain as the nociceptive threshold decreases. Ketamine and its active metabolite, norketamine, are NMDA receptor antagonists with analgesic activities. Low doses of ketamine have been used effectively against neuropathic pain in cases where opioids are not effective. When ketamine is used as an adjuvant analgesic with opioids, it has been shown that less than 50 ng/mL of ketamine exerts effective analgesia without any adverse effects [7, 11, 13, 17].
Ketamine is metabolized by various cytochrome 450 isoforms in the liver and its main metabolite, norketamine, is formed through N-demethylation. Norketamine is only one-third to one-fifth as potent as ketamine in regard to the anesthetic effect. Although the relationship was not evident between the concentration of norketamine and the analgesic effect following administration, it may also have analgesic effects.
Yanagihara et al [18] have suggested that the highest Cmax of ketamine was obtained after administration as a sublingual tablet, while the highest Cmax of norketamine were obtained after administration as a tablet, when 50 mg of ketamine was administered as a sublingual tablets, suppositories, nasal sprays, or tablets. The Cmax of norketamine was 1.1 times higher than the Cmax of ketamine following administration as a nasal spray, approximately two times higher following administration as a sublingual tablet or suppository, and 4.5 times higher following administration in a tablet formulation. Clements [17] reported that the Cmax of norketamine was 0.3 times higher than that of ketamine following administration of ketamine im at a dose of 0.5 mg/kg, while Cmax of norketamine was 4.5 times higher than that of ketamine after oral administration. The Cmax of ketamine was lower, whereas that of norketamine was higher, following oral administration of ketamine as compared to that following administration by other routes.
Ketamine liquid formulation has been demonstrated to provide analgesic effects and been used as a compounded formulation in hospital pharmacies in Japan. In this study, the pharmacokinetics of ketamine and norketamine following administration of the oral liquid formulation at the dose of 50 mg were determined in healthy volunteers. The Cmax of ketamine was 29.9 ± 26.8 ng/mL, while that of norketamine was 250.2 ng/mL, about 8.4 times as high as that of ketamine. In addition, the AUC0-12 of norketamine was 1193.1 ± 159.6 ng hr/mL, 15.1 times higher than that of ketamine. Orally administerd ketamine undergoes extensive first-pass metabolism in the liver, resulting in a bioavailability of approximately 16% [17]. Our findings suggest that norketamine may contribute to the analgesic effect after oral administration of ketamine.
Almost no accumulation of ketamine in the serum was observed following administration of oral liquid ketamine, because T1/2 is 1.1 ± 0.5 hr, which is extremely short, in the healthy volunteers. The T1/2 of norketamine was 5.3 ± 1.1 hr, 4.8 times longer than that of ketamine. Thus, there is a possibility of accumulation of norketamine in patients with renal failure, as norketamine is eliminated by the kidney [19].
None of the subjects experienced any severe side effects after administration of the liquid formulation of ketamine.
Our study was small in scale, and we could not evaluate the effects of the oral formulation of ketamine as an analgesic. The relationship between the effects and concentrations of ketamine/norketamine in patients need to be verified in a future study. The type of ketamine preparation to be administered to a patient should be selected in accordance with the patient’s condition, and use of the liquid formulation may be one of the useful options.
| References | ▴Top |
- Arita H, Hanaoka K. Chronic pain and ketamine. Pain Clinic. 1999;20(8):1134-1141. (Japanese).
- Kato M. Intravenous drip of ketamine for neuropathic pain and cancer pain. Pain Clinic. 2003;24(4):493-499. (Japanese).
- Reich DL, Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36(2):186-197.
doi pubmed - White PF, Johnston RR, Pudwill CR. Interaction of ketamine and halothane in rats. Anesthesiology. 1975;42(2):179-186.
doi pubmed - Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29(11):2396-2399.
doi - Ebert B, Mikkelsen S, Thorkildsen C, Borgbjerg FM. Norketamine, the main metabolite of ketamine, is a non-competitive NMDA receptor antagonist in the rat cortex and spinal cord. Eur J Pharmacol. 1997;333(1):99-104.
doi - Furuhashi-Yonaha A, Iida H, Asano T, Takeda T, Dohi S. Short- and long-term efficacy of oral ketamine in eight chronic-pain patients. Can J Anaesth. 2002;49(8):886-887.
doi pubmed - Eide K, Stubhaug A, Oye I, Breivik H. Continuous subcutaneous administration of the N-methyl-D-aspartic acid (NMDA) receptor antagonist ketamine in the treatment of post-herpetic neuralgia. Pain. 1995;61(2):221-228.
doi - Oshima E, Tei K, Kayazawa H, Urabe N. Continuous subcutaneous injection of ketamine for cancer pain. Can J Anaesth. 1990;37(3):385-386.
doi pubmed - Ushida T, Tani T, Kanbara T, Zinchuk VS, Kawasaki M, Yamamoto H. Analgesic effects of ketamine ointment in patients with complex regional pain syndrome type 1. Reg Anesth Pain Med. 2002;27(5):524-528.
pubmed - Grant IS, Nimmo WS, Clements JA. Pharmacokinetics and analgesic effects of i.m. and oral ketamine. Br J Anaesth. 1981;53(8):805-810.
doi pubmed - Yanagihara Y, Ohtani M, Matsumoto M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, et al. [Preparation of ketamine tablets for treatment of patients with neuropathic pain]. Yakugaku Zasshi. 1999;119(12):980-987.
pubmed - Yanagihara Y. Studies on development and clinical application of ketamine. preparations for neuropathic pain relief. 2006; 32(4): 275-288.
- Kaneuchi M, Sawaguchi R, Kohri N, Senbongi K, Sakai H, Asano M, Iseki K. Evaluation of preparation of ketamine in hospital for practical use -analysis of plasma concentration profiles of ketamine and norketamine after oral and buccal administration -. 2006;13(1):18-22.
- Chong C, Schug SA, Page-Sharp M, Jenkins B, Ilett KF. Development of a sublingual/oral formulation of ketamine for use in neuropathic pain: Preliminary findings from a three-way randomized, crossover study. Clin Drug Investig. 2009;29(5):317-324.
doi pubmed - Bolze S, Boulieu R. HPLC determination of ketamine, norketamine, and dehydronorketamine in plasma with a high-purity reversed-phase sorbent. Clin Chem. 1998;44(3):560-564.
pubmed - Clements JA, Nimmo WS, Grant IS. Bioavailability, pharmacokinetics, and analgesic activity of ketamine in humans. J Pharm Sci. 1982;71(5):539-542.
doi pubmed - Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, Aoyama T, et al. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Biopharm Drug Dispos. 2003;24(1):37-43.
doi pubmed - White PF, Way WL, Trevor AJ. Ketamine—its pharmacology and therapeutic uses. Anesthesiology. 1982;56(2):119-136.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Current Surgery is published by Elmer Press Inc.
